CCl 4 , an important commercial solvent, is prepared by the reaction of Cl 2 (g) with
Question:
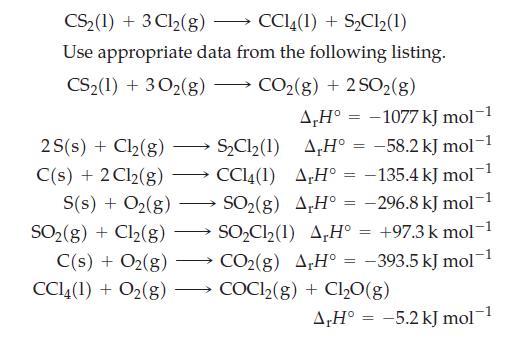
CCl4, an important commercial solvent, is prepared by the reaction of Cl2(g) with a carbon compound. Determine ΔrH° for the reaction
Transcribed Image Text:
CS₂(1) + 3Cl₂(g) CCl4(1) + S₂Cl2 (1) Use appropriate data from the following listing. CS₂(1) + 302(g) CO₂(g) + 2SO2(g) A,H° -1077 kJ mol 2S(s) + Cl₂(g) AH° = -58.2 kJ mol-1 A,H° = -135.4 kJ mol-1 C(s) + 2Cl2(g) S(s) + O₂(g) →SO₂(g) A,H° = -296.8 kJ mol-1 SO₂(g) + Cl₂(g) SO₂Cl₂(1) AH° = +97.3 k mol-1 CO₂(g) AH° = -393.5 kJ mol™ COC1₂(g) + Cl₂O(g) C(s) + O₂(g) CC14 (1) + O₂(g) - → S₂Cl₂(1) CCl4(1) = A,H° -5.2 kJ mol-1 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To determine rH for the reaction of Cl2g with a carbon co...View the full answer

Answered By

Gilbert Chesire
I am a diligent writer who understands the writing conventions used in the industry and with the expertise to produce high quality papers at all times. I love to write plagiarism free work with which the grammar flows perfectly. I write both academics and articles with a lot of enthusiasm. I am always determined to put the interests of my customers before mine so as to build a cohesive environment where we can benefit from each other. I value all my clients and I pay them back by delivering the quality of work they yearn to get.
4.80+
14+ Reviews
49+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The catalyst [Rh(Ph 2 PCH 2 CH 2 PPh 2 )] + can be prepared by the reaction of [Rh(nbd)(Ph 2 PCH 2 CH 2 PPh 2 )] + (nbd = 25.37) with two equivalents of H 2 . In coordinating solvents [Rh(Ph 2 PCH 2...
-
The following are the financial statements of Swifty Corporation. Swifty Corporation Comparative Balance Sheets December 31 Assets 2019 2018 Cash $37,200 $19,700 Accounts receivable 33,000 18,400...
-
Q. 3. a) Given that Bakkar is just new in the market, what would you describe as his best distribution channel? And what marketing options does he have to advertise and popularize his product while...
-
100 grams of R-134a initially fill a weighted piston-cylinder device at 60 kPa and 220oC. The device is then heated until the temperature is 100oC. Determine the change in the device's volume as a...
-
This chapter explained the purpose of managerial accounting in the context of the current business environment. Review the automobile section of your local newspaper; the Sunday paper is often best....
-
In the late 1970s, a large part of athletic shoe production shifted from plants in the United States to plants in South Korea. In late 1993, it was reported that South Korean shoe firms had suffered...
-
MIRR Refer to Problem 11-1. What is the projects MIRR? AppendixlLO1
-
The Redford Corporation took out a 20-year mortgage on June 15, 2011, for $2,600,000 and pledged its only manufacturing building and the land on which the building stands as collateral. Each month...
-
1) A firm is considering an investment in a new manufacturing plant. The site already is owned by the company, but existing buildings would need to be demolished. Which of the following should be...
-
Implement the Following and check the errors public class MyLinkedList { static class Node { String value; Node next; Node(String value, Node next) { this.value = value; ...
-
Use Hesss law and the following data CH4(g) + 2O2(g) CH4(g) + CO(g) CH(g) + HO(g) CO(g) + 2HO(g) A,H -802 kJ mol 2 CO(g) + 2H(g) A,H = +247 kJ mol-1 CO(g) + 3H(g) A,H +206 kJ mol1 to determine A,H...
-
Substitute natural gas (SNG) is a gaseous mixture containing CH 4 (g) that can be used as a fuel. One reaction for the production of SNG is 4 CO(g) + 8 H(g) 3 CH4(g) + CO(g) + 2 HO(1) A,H = ? Use...
-
The Frame Division of Reliable Computers manufactures storage housings for personal and Obj. 7 midsize computers, which it sells externally to computer manufacturers. The division is operating at...
-
FICO credit scores: x = 564,= 743,= 72 (Round your answer to 3 decimal places.) what does z equal
-
Q3: In the section illustrated in Figure (1) the surface 1-4-7 is insulated. The convection heat transfer coefficient at surface 1-2-3 is 28 W/m. 'C. The thermal conductivity of the solid material is...
-
25 of 27 > This test: 96 point(s) possible This question: 3 point(s) possible Submit test Identical twins come from a single egg that split into two embryos, and fraternal twins are from separate...
-
The figure shows a turbine-driven pump that provides water, at high pressure, to a tank located 25-m higher than the pump. Steady-state operating data for the turbine and the pump are labelled on the...
-
Step 1 Step 2 1. Sketch what step 4 and then step 5 would look like. Step 4 Step S 2. How many black triangles are in each step? Step 1 black A = | Step 2 = 4 black A's step 3 = 13 black D's 3. What...
-
Why does a TiN-coated tool consume less power than an un-coated HSS under exactly the same cutting conditions?
-
If a force of F = 50 Ib is applied to the pads at A and C, determine the smallest dimension d required for equilibrium if the spring has an unstretched length of 1 ft. B 1 ft 1 ft F k = 15016/fr 1ft...
-
Briefly describe some of the similarities and differences between U.S. GAAP and iGAAP with respect to reporting accounting changes.
-
How might differences in presentation of comparative data under U.S. and iGAAP affect adoption of iGAAP by U.S. companies?
-
Wertz Construction Company decided at the beginning of 2010 to change from the completed contract method to the percentage-of-completion method for financial reporting purposes. The company will...
-
Sierra Company manufactures soccer balls in two sequential processesCutting and Sutching. All direct materials enter production at the beginning of the cutting process. The following information is...
-
Whenever alimony and child support payments are both required as the result of a divorce decree and the full payment is not made, child support is always considered to have been paid first. Choose...
-
MAJOR CASE STUDY You have commenced work at Alfreds Accountants, and Alfred has given you a series of tasks to perform. The first task is as follows: Alfred hands you a pre-adjustment trial balance...

Study smarter with the SolutionInn App


