Substitute natural gas (SNG) is a gaseous mixture containing CH 4 (g) that can be used as
Question:
Substitute natural gas (SNG) is a gaseous mixture containing CH4(g) that can be used as a fuel. One reaction for the production of SNG is
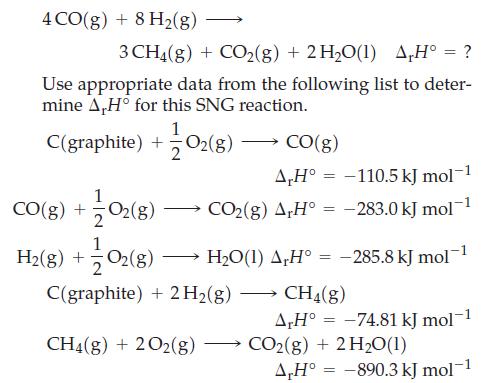
Transcribed Image Text:
4 CO(g) + 8 H₂(g) 3 CH4(g) + CO₂(g) + 2 H₂O(1) A,H° = ? Use appropriate data from the following list to deter- mine A,H° for this SNG reaction. C(graphite) + O₂(g) CO(g) + O₂(g) H₂(g) + O₂(g) CO(g) A,Hº -110.5 kJ mol-1 CO₂(g) AH = -283.0 kJ mol-¹ -285.8 kJ mol-¹ H_O(1) A,H° C(graphite) + 2 H₂(g) CH4(g) + 2O2(g) = = CH4(g) A,H° -74.81 kJ mol-1 = CO2(g) + 2 H₂0(1) A,H° -890.3 kJ mol-1 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
To determine the standard enthalpy of formation of SNG from the given reaction we can use Hesss Law ...View the full answer

Answered By

Khurram shahzad
I am an experienced tutor and have more than 7 years’ experience in the field of tutoring. My areas of expertise are Technology, statistics tasks I also tutor in Social Sciences, Humanities, Marketing, Project Management, Geology, Earth Sciences, Life Sciences, Computer Sciences, Physics, Psychology, Law Engineering, Media Studies, IR and many others.
I have been writing blogs, Tech news article, and listicles for American and UK based websites.
4.90+
5+ Reviews
17+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A gaseous mixture containing 1.00 mol each of CO, H2O, CO2, and H2 is exposed to a zinc oxidecopper oxide catalyst at 1000oC. The reaction is and the equilibrium constant Kc is 0.58 at 1000oC. What...
-
Ammonia is synthesized from hydrogen and nitrogen. The synthesis gas is usually produced from hydrocarbons. The most common raw materials are oil or natural gas, though coal and even peat can be...
-
Steam reforming is an important technology for converting refined natural gas, which we take here to be methane, into a synthesis gas that can be used to produce a variety of other chemical...
-
Consider Southeast Home Care, which is a for-profit business. In 2020, its net income was $1,500,000 and it distributed $500,000 to owners in the form of dividends. Its beginning-of-year equity...
-
Fraud affects Best Buy. Refer to Best Buys financial statements in Appendix A to answer the following: 1. Explain how inventory losses (such as theft) impact how Best Buy reports inventory on its...
-
There was sluggishness in the Japanese economy in the 1990s, and Japans terms of trade improved at the same time. Can you interpret and analyze this experience in the context of what you have studied...
-
IRR Refer to Problem 11-1. What is the projects IRR? AppendixlLO1
-
At December 31, 2015, certain accounts included in the property, plant, and equipment section of Townsand Companys balance sheet had the following balances: Land............ $ 100,000...
-
Listed below are the transactions of Yasunari Kawabata, D.D.S., for the month of September. Sept. 1 Kawabata begins practice as a dentist, invests $20,000 cash and issues 2,000 shares of $10 par...
-
The Town of Weston has a Water Utility Fund with the following trial balance as of July 1, 2019, the first day of the fiscal year: Credits Cashi Customer accounts receivable Allowance for...
-
CCl 4 , an important commercial solvent, is prepared by the reaction of Cl 2 (g) with a carbon compound. Determine r H for the reaction CS(1) + 3Cl(g) CCl4(1) + SCl2 (1) Use appropriate data from...
-
Determine r H for this reaction from the data below. NH4(1) + 2HO2(1) NH4(1) + O2(g) H(g) + O2(g) 1 2 H(g) + O(g) N(g) + 4H0(1) N(g) + 2 H0(1) A,H -622.2 kJ mol-1 -285.8 kJ mol-1 -187.8 kJ mol- =...
-
Mott Company recently implemented a JIT manufacturing system. After one year of operation, Heidi Burrows, president of the company, wanted to compare product cost under the JIT system with product...
-
The pressure cooker pictured here consists of a light pressure vessel with a heavy lid of weight W. When the lid is secured, the vessel is filled with a hot pressurized gas of pressure p. After some...
-
5) A large group of students took a test in Finite Math where the grades had a mean of 72 and a standard deviation of 4. Assume that the distribution of these grades is approximated by a normal...
-
Q9 (5 points) According to Dr. Henry Mintzberg, a noted management scholar from McGill University in Montreal, PQ, "business organizations perform only two activities of consequence." What are these...
-
Q3: In the section illustrated in Figure (1) the surface 1-4-7 is insulated. The convection heat transfer coefficient at surface 1-2-3 is 28W / (m ^ 2) ."C. The thermal conductivity of the solid...
-
For the data given in Table 23.1, use a graphics package or a spreadsheet, such as Excel, to construct the following graphs: a. Pie chart b. Line chart c. Bar chart (lb) (5b) (5c 6a (la) TABLE 23.1...
-
What are the most common surface treatments for a. High-speed steels? b. Carbides? c. Ceramics?
-
Construct a 4 x 25 design confounded in two blocks of 16 observations each. Outline the analysis of variance for this design.
-
Refer to the accounting change by Wertz Construction Company in BE22-1. Wertz has a profit-sharing plan, which pays all employees a bonus at year-end based on 1% of pre-tax income. Compute the...
-
Shannon, Inc., changed from the LIFO cost flow assumption to the FIFO cost flow assumption in 2010. The increase in the prior years income before taxes is $1,200,000. The tax rate is 40%. Prepare...
-
Tedesco Company changed depreciation methods in 2010 from double-declining-balance to straight-line. depreciation prior to 2010 under double-declining-balance was $90,000, whereas straight line...
-
The existence of cognitive biases eliminates the need for a proper application of professional skepticism on audits. True or False
-
Answer question D) only! A) C urrent co ting system charge overhead to predects based a direct laber cut wig single planter. What products w repertlar the precis here Overhead te perdoria) (100 000...
-
On January 1, 20X1, Toy inc. issued $500,000 of convertible bonds. The bonds mature on December 31, 20X5. Interest is payable annually at 6.0% on December 31. The bonds are convertible at the...

Study smarter with the SolutionInn App


