Determine r H for this reaction from the data below. NH4(1) + 2HO2(1) NH4(1) + O2(g)
Question:
Determine ΔrH° for this reaction from the data below.
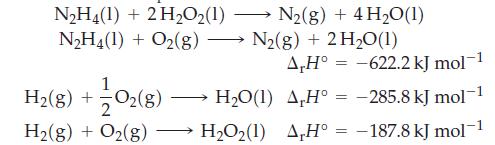
Transcribed Image Text:
N₂H4(1) + 2H₂O2(1) N₂H4(1) + O2(g) H₂(g) + O2(g) 1 2 H₂(g) + O₂(g) N₂(g) + 4H₂0(1) N₂(g) + 2 H₂0(1) A,H° -622.2 kJ mol-1 -285.8 kJ mol-1 -187.8 kJ mol-¹ = H₂O(1) A,H° = H₂O₂(1) AH° =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
To determine rH for the reactionwe can use the followi...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A 1.103 g sample of a gaseous carbonhydrogenoxygen compound that occupies a volume of 582 mL at 765.5 Torr and 25.00 C is burned in an excess of O 2 (g) in a bomb calorimeter. The products of the...
-
The Internet has been a major disruptive force in modern retailing, helping bring about the demise of numerous familiar stores in your local mall: The Limited, American Apparel, Wet Seal,...
-
Required: Hemming uses a perpetual inventory system. 1. Determine the costs assigned to ending inventory and to cost of goods sold using FIFO. 2. Determine the costs assigned to ending inventory and...
-
Describe the value paradox: the economics of diamonds and water.
-
The following chart shows how costs flow through a business as a product is manufactured. Some boxes in the flowchart show cost amounts. Compute the cost amounts for the boxes that contain...
-
Using the example in Question 1, how does an equivalent subsidy to the import-competing producer affect the market? What is the cost to the government of this subsidy? Which policy would consumers...
-
NPV Project K costs $52,125, its expected cash inflows are $12,000 per year for 8 years, and its WACC is 12%. What is the projects NPV? AppendixlLO1
-
Maggies Skunk Removal Corp.s 2015 income statement listed net sales = $12.5 million, gross profit of $6.9 million, EBIT = $5.6 million, net income available to common stockholders = $3.2 million, and...
-
Part Pacific Spirit Corp. borrowed S 100,000 in the form of a mortgage on January 1, 2017, to finance the purchase of a small warehouse. The mortgage rate is 5 percent and the term 10 years, and...
-
Stockman, Turbo and United are three small firms producing specialized products, equipment, and research for the scientific and medical communities. As this has become such a critical, high-growth...
-
Substitute natural gas (SNG) is a gaseous mixture containing CH 4 (g) that can be used as a fuel. One reaction for the production of SNG is 4 CO(g) + 8 H(g) 3 CH4(g) + CO(g) + 2 HO(1) A,H = ? Use...
-
Given the following information: 3 N(g) + -H(g) NH3(g) A,Hi 3 NO(g) + HO(1) A,H2 HO(1) A,H3 Determine A,H for the following reaction, expressed in terms of A,Hi, A,H2, and A,H. N(g) + O(g) 2 NO(g)...
-
. Making journal entries to record and transfer costs. Using your solution to Problem 17-2, give entries in general journal form to record the costs charged to production in the Forming Department...
-
Nike is one of the world's largest and best-known global corporations. Visit the company's website. Where is the company headquartered? What are its products? Where are they manufactured? As a global...
-
A multi-plate clutch of alternate bronze and steel plates having effective diameters of \(175 \mathrm{~mm}\) and \(72.5 \mathrm{~mm}\) has to transmit \(25 \mathrm{~kW}\) at \(2000 \mathrm{rpm}\)....
-
Consider Figure 9-9, panel (b). Based on the data there, which regions support the convergence hypothesis? Which do not? Explain. FIGURE 9-9 Do Economies Converge? (b) But Not for the World as a...
-
Draw a diagram with AD, SRAS and LRAS. Be careful to label the axes correctly.
-
Keeping the settings of the Dang-Gorton-Holmstrm-Ordoez model mostly unchanged, except that - The bank is the only financial firm in the economy, that provides a mixture of deposit contract and bond...
-
Why are multiple coats or layers put on the carbide base for coated tools?
-
In Problems, solve each system of equations. x + 2y + 3z = 5 y + 11z = 21 5y + 9z = 13
-
Sesame Company purchased a computer system for $74,000 on January 1, 2009. It was depreciated based on a 7-year life and an $18,000 salvage value . On January 1, 2011, Sesame revised these estimates...
-
In 2010, Bailey Corporation discovered that equipment purchased on January 1, 2008, for $50,000 was expensed at that time. The equipment should have been depreciated over 5 years, with no salvage...
-
At January 1, 2010, Beidler Company reported retained earnings of $2,000,000. In 2010, Beidler discovered that 2009 depreciation expense was understated by $400,000. In 2010, net income was $900,000...
-
A bond issued on February 1, 2004 with face value of $35800 has semiannual coupons of 6.5%, and can be redeemed for par (face value) on February 1, 2025. What is the accrued interest and the market...
-
Toxaway Company is a merchandiser that segments its business into two divisions-Commercial and Residential. The company's accounting intern was asked to prepare segmented income statements that the...
-
The local supermarket is considering investing in or Checkout kioske for its customers. The wol-checkout kiosks will cost $47.000 and have no residual van Management expects the equipment to rest in...

Study smarter with the SolutionInn App


