Use Hesss law to determine r H for the reaction C 3 H 4 (g) +
Question:
Use Hess’s law to determine ΔrH° for the reaction C3H4(g) + 2 H2(g) → C3H8(g), given that
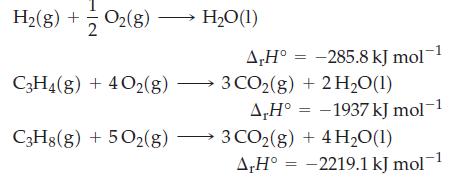
Transcribed Image Text:
H₂(g) + O2(8) O₂(g) → C3H4(g) + 4O2(g) C3H8(g) + 5O2(g) H₂O(1) A,H° -285.8 kJ mol-1 3 CO2(g) + 2H₂O(1) A,H° -1937 kJ mol-1 3 CO2(g) + 4H₂O(1) A,H° -2219.1 kJ mol-1 = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Using Hesss law we can determine the enthalpy of reaction for ...View the full answer

Answered By

Vikash Gupta
I am graduated in Physics in 2018, from KIRORIMAL COLLEGE, University of Delhi. Now I am persuing Master's degree in physics. I like to do physics problems. I have experience of 1 year in tutoring. I think Physics is the only subject where you understand things,how they are happening . In physics you learn Maths and apply it. So I would like to join your platform to solve many Physics problems.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The gas-phase reaction between Br2 and H2 to form HBr is assumed to proceed by the following mechanism: a. Under what conditions does the rate law have the form rate = k[Br2]? b. Under what...
-
The equilibrium constant for the reaction H2 + at 1 atm and 1500C is given to be K. Of the reactions given below, all at 1500C, the reaction that has a different equilibrium constant is (a) H2 + 12O2...
-
The decomposition of NH3 to N2 and H2 was studied on two surfaces: Without a catalyst, the activation energy is 335 kJ/ mol. a. Which surface is the better heterogeneous catalyst for the...
-
A heat engine operates between two reservoirs at 800 and 20C. One-half of the work output of the heat engine is used to drive a Carnot heat pump that removes heat from the cold surroundings at 2C and...
-
Given the following selected account balances of Spalding Company, prepare its manufacturing statement for the year ended on December 31, 2009. Include a listing of the individual overhead account...
-
Suppose country A is considering forming a customs union with country B. Country A produces only manufactured goods and imports all its raw materials and agricultural products. Country B produces...
-
What reinvestment rate assumptions are built into the NPV, IRR, and MIRR methods? Give an explanation for your answer. AppendixlLO1
-
The Houston Corp needs to raise money for an addition to its plant. It will issue 300,000 shares of new common stock. The new shares will be priced at $60 per share with an 8.5% spread on the offer...
-
An owner investment of a building, valued at $200,000, along with a $55,000 outstanding mortgage, into an entity would. O a. decrease liabilities $145,000. O b. increase owner's equity $200,000. c....
-
By the Month Inc. sold 32,000 annual magazine subscriptions for $10 during December 20Y4. These new subscribers will receive monthly issues, beginning in January 20Y5. By the Month Inc. issued a...
-
Given the following information: 3 N(g) + -H(g) NH3(g) A,Hi 3 NO(g) + HO(1) A,H2 HO(1) A,H3 Determine A,H for the following reaction, expressed in terms of A,Hi, A,H2, and A,H. N(g) + O(g) 2 NO(g)...
-
Use Hesss law to determine r H for the reaction 1 CO(g) + O2(g) CO(g), given that C(graphite) + +10(8) C(graphite) + O(g) CO(g) A,H -110.54 kJ mol-1 CO(g) A,H = -393.51 kJ mol-1 =
-
Based on the data presented in Exercise 25-18, assume that Smart Stream Inc. uses the variable cost concept of applying the cost-plus approach to product pricing. a. Determine the variable costs and...
-
In given two linked lists. We have to find whether the data in one is reverse that of data in another. No extra space should be used and traverse the linked lists only once. //Sorts a given list by...
-
The graph shows the number of deaths in the United States due to accidents. Answer the following questions about the graph. 1. Name the variables used in the graph. 2. Are the variables qualitative...
-
Tobias Company purchased inventory on account. This transaction will affect: a. Only the balance sheet b. Only the income statement c. The income statement and the statement of retained earnings d....
-
Mat Company's cost of goods sold for the month ended March \(31,20 X X\), was \(\$ 345,000\). Ending work-in-process inventory was \(90 \%\) of beginning work-in-process inventory. Factory overhead...
-
Draw a bar graph for each data set in Problems 32-35. Data set \(\mathrm{A}\) Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 35 25 25 16 14 1...
-
What is the DCL?
-
In a certain school district, 3% of the faculty use none of their sick days in a school year. Find the probability that 5 faculty members selected at random used no sick days in a given year.
-
Simmons Corporation owns stock of Armstrong, Inc prior to 2010 the investment was accounted for using the equity method. In early 2010, Simmons sold part of its investment in Armstrong, and began...
-
Oliver Corporation has owned stock of Conrad Corporation since 2007. At December 31, 2010, its balances related to this investment were: Available-for-Sale Securities $185,000 Securities Fair Value...
-
Change in PrincipleLong-Term Contracts Cherokee Construction Company changed from the completed-contract to the percentage-of-completion method of accounting for long-term construction contracts...
-
Exercise 18-11 (Algo) Balance sheet identification and preparation LO P1 End-of-year current assets for two different companies follow. One is a manufacturer, Rayzer Skis Manufacturing, and the...
-
The duties or restrictions stated in Circular 230, Subpart B, do NOT include __________. Advertising that taxpayers may use pay stubs in lieu of Form W-2. Charging exorbitant fees. Knowingly...
-
Given the table of transactions below, determine the average daily balance of the credit card for the April 1 - April 30 billing period? Day Activity Adjustment End-of-Day Balance 1 ----- ----- 1 ,...

Study smarter with the SolutionInn App


