Use Hesss law to determine r H for the reaction 1 CO(g) + O2(g) CO(g),
Question:
Use Hess’s law to determine ΔrH° for the reaction
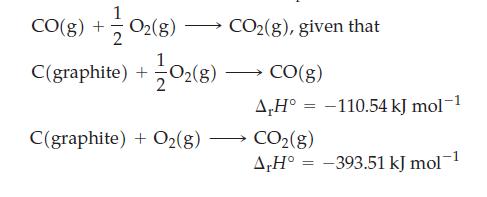
Transcribed Image Text:
1 CO(g) + O2(g) →→→→ CO₂(g), given that C(graphite) + +10₂(8) C(graphite) + O₂(g) CO(g) A,H° -110.54 kJ mol-1 CO₂(g) A,H° = -393.51 kJ mol-1 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
To use Hesss law to determine rH for the reaction Cgraphite O2g CO2g we ...View the full answer

Answered By

Issa Shikuku
I have vast experience of four years in academic and content writing with quality understanding of APA, MLA, Harvard and Chicago formats. I am a dedicated tutor willing to hep prepare outlines, drafts or find sources in every way possible. I strive to make sure my clients follow assignment instructions and meet the rubric criteria by undertaking extensive research to develop perfect drafts and outlines. I do this by ensuring that i am always punctual and deliver quality work.
5.00+
6+ Reviews
13+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Gebruik Hess se Wet om die reaksiewarmte vir die proses te bepaal. Use Hess's Law to determine the heat of reaction for the process. CH4(g) + 6HCl(g) 2CHCl3(g) + 4H(g) i. 2C(s) + 2H(g) CH4(g) | AH =...
-
The purpose of ERISA is to create transparency, accountability, and prevent the mismanagement of the investments made by participants in Plans. This week we are examining the requisite requirements...
-
Funtime, Inc., manufactures video game machines. Market saturation and technological innovations caused pricing pressures that resulted in declining profits. To stem the slide in profits until new...
-
A 16 lb weight is attached to a spring with a spring constant equal to 2 lb/ft. Neglect damping. The weight is released from rest at 3 ft below the equilibrium position. At t = 2r sec, it is struck...
-
For each of the following account balances for a manufacturing company, place a ( in the appropriate column indicating that it appears on the balance sheet, the income statement, the manufacturing...
-
A dollar appreciation against the Swiss franc is no guarantee that the dollar will go further than it previously did in acquiring Swiss goods. Do you agree? Explain.
-
Project X is very risky and has an NPV of $3 million. Project Y is very safe and has an NPV of $2 5 million. They are mutually exclusive, and project risk has been properly considered in the NPV...
-
Terry Lemay is unclear as to the difference between the balance sheets of a merchandising company and a manufacturing company. Explain the difference to Terry.
-
In the manufacture of 9,200 units of a product, direct materials cost incurred was $175,100, direct labor cost incurred was $114,400, and applied factory overhead was $47,900. What is the total...
-
James House is planning on starting a cleaning services business but has not decided whether he should focus on residential clients or commercial clients. His estimates of revenue, variable expenses,...
-
Use Hesss law to determine r H for the reaction C 3 H 4 (g) + 2 H 2 (g) C 3 H 8 (g), given that H(g) + O2(8) O(g) C3H4(g) + 4O2(g) C3H8(g) + 5O2(g) HO(1) A,H -285.8 kJ mol-1 3 CO2(g) + 2HO(1) A,H...
-
Write an equation to represent the combustion of thymol referred to in Exercise 44. Include in this equation the values for U and H. Exercise 44 A 1.397 g sample of thymol, C 10 H 14 O(s) (a...
-
Operating cash flow per share is a better indicator of profitability than is earnings per share. Do you agree? Explain.
-
a. A farmer friend of yours is going to build his own distillation system to purify ethanol made by fermentation. He wants to make his own packing. Suggest 30 different things he could make or buy...
-
In Table 15-5, why does customer service speed of performance increase faster for customers located greater distances from a warehouse facility? What is the implication of this relationship for...
-
For each of the accounts listed below, indicate whether the account is increased by a debit or a credit: Accounts Payable Advertising Expense Cash Common Stock Dividends Equipment Land Service Fees...
-
The battle for leadership was set. Topps Trading Cards had been producing baseball cards for more than 50 years. New Yorkbased Topps was ready to accept an offer from Michael Eisners Tornante Co. and...
-
For each of the accounts listed below, indicate whether the account is increased by a debit or a credit: Accounts Receivable Advertising Revenue Building Common Stock Notes Payable Retained Earnings...
-
What is a chip groove?
-
Write the given system without the use of matrices. D) - ()- d (x sin t + 8 (2+ 1)
-
Change in PrincipleInventory Methods Whitman Company began operations on January 1, 2008, and uses the average cost method of pricing inventory. Management is contemplating a change in inventory...
-
Accounting Change Ramirez Co. decides at the beginning of 2010 to adopt the FIFO method of inventory valuation. Ramirez had used the LIFO method for financial reporting since its inception on January...
-
Accounting Change Linden Company started operations on January 1, 2006, and has used the FIFO method of inventory valuation since its inception. In 2012, it decides to switch to the average cost...
-
please check all paper Completing Your Assignment What am I required to do in this assignment? Introduction Capital Avestment appraisal is concerned with organizational management decisions about...
-
Patricia owns 20% of a partnership that reported net income of $130,000 for the year. During the year $18,000 was distributed to Patricia from the partnership. How much should Patricia include in her...
-
2. Auditors are required to obtain a sufficient understanding of each of the elements of an entity's internal control (environment, accounting system, control policies and procedures). This...

Study smarter with the SolutionInn App


