Question: Use the following electrode potential diagram for basic solutions to classify each of the statements below as true or false. Assume standard conditions. (a) Sulfate
Use the following electrode potential diagram for basic solutions to classify each of the statements below as true or false. Assume standard conditions.
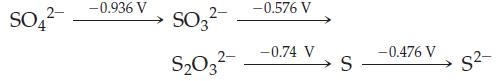
(a) Sulfate (SO42–) is a stronger oxidant than thiosulfate (S2O32–) in basic solution.
(b) S2– can be used as a reducing agent in basic solutions.
(c) S2O32– is stable with respect to disproportionation to SO32– and S in basic solution.
SO4 -0.936 V SO3- S03- -0.576 V -0.74 V S -0.476 V S-
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
a False Explanation The electrode potential diagram shows that the standard reduction potential of S... View full answer

Get step-by-step solutions from verified subject matter experts


