Use the unit cell of diamond in Figure 12-32(b) and a carbon-to-carbon bond length of 154.45 pm,
Question:
Use the unit cell of diamond in Figure 12-32(b) and a carbon-to-carbon bond length of 154.45 pm, together with other relevant data from the text, to calculate the density of diamond.
Figure 12-32(b)
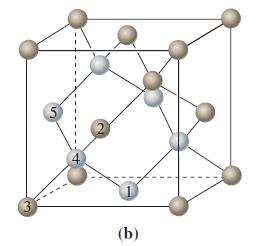
Transcribed Image Text:
(b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Calculating the density of diamond from the unit cell in Figure 1232b The unit cell of diamond is a ...View the full answer

Answered By

Samananda Khangembam
At present I do research in astrophysics at a university. Earlier I taught 10+2 physics at 2 premier Senior Secondary Schools in the state on Manipur in India. Besides, I also taught physics for test preparation for entry to medical and engineering schools in India. All together I have more than 5 years of experience in teaching physics at K2 level. I were also a teaching assistant for undergraduate studies at Northern Illoinois University(NIU), USA at the Department of Physics.
0.00
0 Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The edge length of the unit cell of tantalum metal, Ta, is 330.6 pm; the unit cell is body centered cubic (one atom at each lattice point). Tantalum has a density of 16.69 g/cm3. What is the mass of...
-
A diamond unit cell is shown here. (a) How many carbon atoms are in one unit cell? (b) The unit cell can be considered as a cubic unit cell of C atoms with other C atoms in holes in the lattice. What...
-
Bugsy provided the following operating information during the current year. Operating income $ 185,000 Selling, general, and administrative costs 42,000 Cost of goods sold 77,500 Required: Provide...
-
In the previous problem, assume the equity increases by 1,500 solaris due to retained earnings. If the exchange rate at the end of the year is 1.24 solaris per dollar, what does the balance sheet...
-
Eldons Super Fresh Stores, Inc., is a corporation engaged in the retail grocery business. William Drexler was the attorney for and the corporate secretary of Eldons and was also the personal attorney...
-
Using the information of Gloster Udy at the end of June 2022 (shown in figure 6.93) and the transactions for July: enter the appropriate journal abbreviation next to each transaction prepare the...
-
How can community-based social enterprises ensure that their social mission can be attained?
-
Multiple Choice Questions The following questions concern CPA firms' liability under common law. Choose the best response. a. Sharp, CPA, was engaged by Peters & Sons, a partnership, to give an...
-
You want to buy a new sports coupe for $88,500, and the finance office at the dealership has quoted you an APR of 7 percent for a 72 month loan to buy the car. a. What will your monthly payments be?...
-
Identify the compound with molecular formula C7H14O that gives the following proton-coupled 13C NMR spectrum. 80 60 40 20 o (ppm) frequency
-
The enthalpy of formation of NaI(s) is -288 kJ mol -1 . Use this value, together with other data in the text, to calculate the lattice energy of NaI(s).
-
One way to describe ionic crystal structures is in terms of cations filling voids among closely packed anions. Show that in order for cations to fill the tetrahedral voids in a close packed...
-
2-Bromo-1,3-dimethylbenzene is inert to nucleophilic aromatic substitution on treatment with sodium amide in liquid ammonia. It is recovered unchanged even after extended contact with the reagent....
-
A variable mesh screen produces a linear and axisymmetric velocity profile as indicated below in the air flow through a 2-ft diameter circular cross section duct. The static pressures upstream and...
-
A vertical round steel rod 2 m long is securely held at its upper end. A weight can slide freely on the rod and its fall is arrested by a stop provided at the lower end of the rod. When the weight...
-
8) Determine the magnitudes of the forces F and P so that the single equivalent couple (i.e. the resultant of the three couples) acting on the triangular block is zero. Z -F F 3 m 10 N, 30 6 m 10 N 3...
-
PP Company purchases a material that is then processed to yield three chemicals: anarol, estyl, and betryl.In June, PPC purchased 10,000 gallons of the material at a cost of $250,000, and the company...
-
Suppose Boyson Inc. free cash flow for the next year is $ 1 5 0 , 0 0 0 and the FCF is expected to grow a concert rate of 6 . 5 % if WACC is 1 2 . 5 % what is the market value of the firm?
-
Write an equation for the reaction of pyridine with a. cold sulfuric acid (H2SO4) b. cold nitric acid (HNO3)
-
You continue to work in the corporate office for a nationwide convenience store franchise that operates nearly 10,000 stores. The per- store daily customer count (i.e., the mean number of customers...
-
Statement of Cash Flows Suppose a company lengthens the time it takes to pay suppliers. How would this affect the statement of cash flows? How sustainable is the change in cash flows from this...
-
Calculating Liquidity Ratios SDJ, Inc., has net working capital of $l,570 current liabilities of $4,380, and inventory of $1,875. What is the current ratio? What is the quick ratio?
-
Calculating Profitability Ratios Country Boy, Inc., has sales of $24 million, total assets of $18 mil lion, and total debt of $7 million. If the profit margin is 8 percent, what is net income? What...
-
1) A portfolio consists of 3 securities have the following characteristics in terms of standard deviation, proportion of investment and correlation coefficient. Security Standard deviation...
-
Find the future values of the ordinary annuities at the given annual rate r compounded as indicated. The payments are made to coincide with the periods of compounding. (Round your answer to the...
-
Your company has preferred stock currently selling for $65.24 on the New York Stock Exchange (NYSE). If the stock paid a dividend of $4.8 last year, what is the cost of preferred stock to your...

Study smarter with the SolutionInn App


