Question: Use thermodynamic data from Appendix D to calculate a theoretical voltage of the silverzinc button cell described on page 893. TABLE D.1 Ground-State Electron Configurations
Use thermodynamic data from Appendix D to calculate a theoretical voltage of the silver–zinc button cell described on page 893.
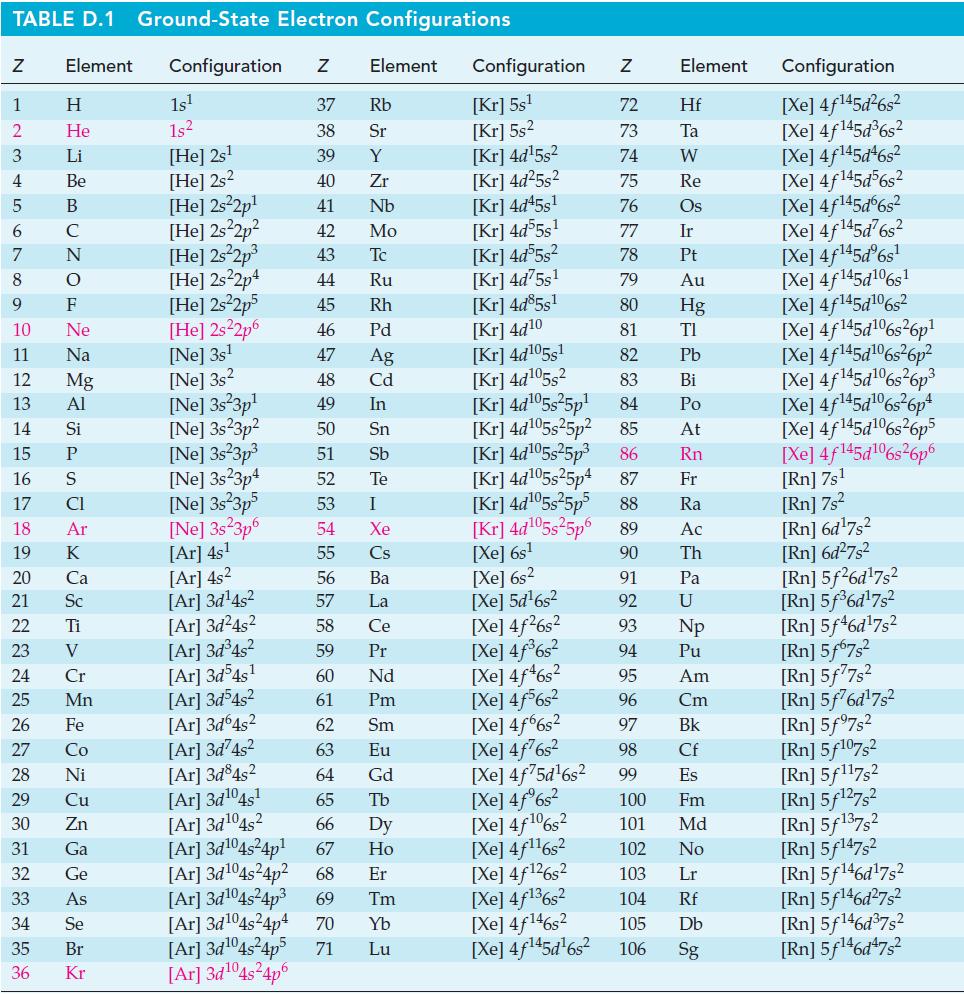
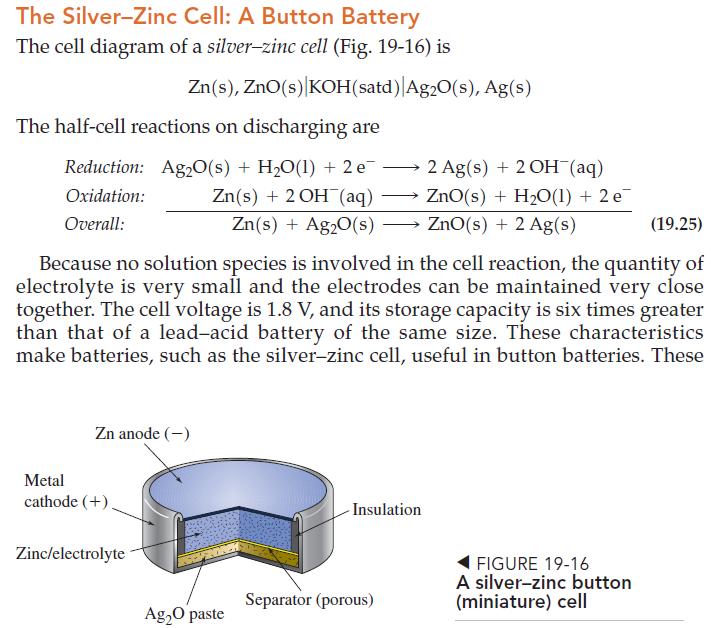
TABLE D.1 Ground-State Electron Configurations Element Configuration Z Z 1 2 3 4 5 6 7 8 9 HIG&LUZONSUZ SE> 0 2 3 2 3 5 3 3 2 2 5 2 He 10 11 12 13 14 15 16 17 18 19 20 21 Sc 22 23 24 25 Mn Mg 26 27 28 Ni 29 30 31 32 33 34 35 36 Zn Ga Ge 1s 1s [He] 2s [He] 2s2 [He] 2s2p [He] 2s2p [He] 2s2p [He] 2s22p4 [He] 2s2p5 [He] 2s2p6 [Ne] 3s [Ne] 3s2 [Ne] 3s 3p [Ne] 3s23p 37 Rb 38 Sr 39 Y 40 Zr 41 Nb 42 Mo 43 Tc 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 Sn 51 Sb 52 Te 53 54 55 [Ar] 4s 56 [Ar]3d4s 57 [Ar]3d4s 58 [Ar]3d4s 59 [Ar]3d54s 60 61 [Ar]3d4s [Ar]3d64s 62 [Ar]3d4s 63 [Ar]3d845 64 [Ar]3d04s 65 [Ar]3d04s2 [Ar]3d04s4p [Ar]3d04s4p [Ar]3d04s4p 69 Tm 66 Dy 67 Ho 68 Er [Ar]3d04s4p4 70 Yb Lu [Ar]3d04s4p5 71 [Ar]3d04s4p6 [Ne] 3s3p [Ne] 3s23p4 [Ne] 3s3p5 Element [Ne] 3s 3p6 [Ar] 4s I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Configuration Z [Kr] 5s [Kr] 5s [Kr] 4d5s [Kr] 4d5s [Kr] 4d45s [Kr] 4d55s [Kr] 4d55s [Kr] 4d75s [Kr] 4d85s1 [Kr] 4d10 [Kr] 4d105s1 [Kr] 4d05s [Kr] 4d05s5p [Kr] 4d05s25p [Kr] 4d05s5p [Kr] 4d05s25p4 [Xe] 6s [Xe] 5d6s [Xe] 4f6s [Xe] 4f6s [Xe] 4f46s2 [Xe] 4f6s2 [Xe] 4f6s2 [Xe] 4f6s [Xe] 4f75d6s [Xe] 4f%s2 [Xe] 4f106s2 [Xe] 4f6s NRNKERKR [Xe] 4f126s2 [Xe] 4f136s2 [Xe] 4f146s2 [Xe] 4f45d6s 72 Hf 73 Ta W 74 75 Re 76 Os 77 Element 78 79 80 81 82 83 84 85 86 87 Fr [Kr] 4d05s5p5 88 Ra [Kr] 4d05s5p6 89 Ac [Xe] 6s 90 Th 91 92 93 94 95 96 97 98 99 Ir Pt Au Hg TI Pb Bi Po At Rn Pa U Np Pu Am Cm Bk Cf Es 100 Fm 101 Md 102 No 103 Lr 104 Rf 105 Db 106 Sg Configuration [Xe] 4f45d6s [Xe] 4f145d6s [Xe] 4f145d46s2 [Xe] 4f145d56s2 [Xe] 4f145d6s2 [Xe] 4f45d6s [Xe] 4f45d6s [Xe] 4f145d106s1 [Xe] 4f145d106s2 [Xe] 4f145d6s26p* [Xe] 4f145d106s36p? [Xe] 4f145d16s?6p3 [Xe] 4f145d6s6p* [Xe] 4f145d16s26p5 [Xe] 4f145d106s26p6 [Rn] 7s [Rn] 7s [Rn] 6d7s [Rn] 6d7s [Rn] 5f26d7s [Rn] 5f6d7s [Rn] 5f46d7s2 [Rn] 5f67s [Rn] 5f77s [Rn] 5f76d7s [Rn] 5f97s2 [Rn] 5f107,2 [Rn] 5f117s2 [Rn] 5f27s [Rn] 5f137,2 [Rn] 5f147s2 Rn] 5f46d7s [Rn] 5f46d7s [Rn] 5f46d7s2 [Rn] 5f46d7s
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


