Verify that a 20% aqueous solution by mass of sucrose (C 12 H 22 O 11 )
Question:
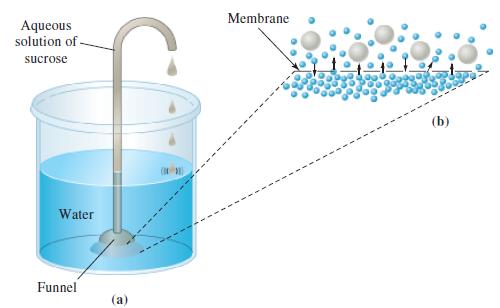
Verify that a 20% aqueous solution by mass of sucrose (C12H22O11) would rise to a height of about 150 m in an apparatus of the type pictured in Figure 14-21.
Figure 14-21
Transcribed Image Text:
Aqueous solution of sucrose Water Funnel (a) Membrane (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Verifying that a 20 aqueous solution by mass of sucrose would rise to a height of about 150 m in an apparatus of the type pictured in Figure 1421 Assumptions The apparatus is semipermeable meaning tha...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A 19.2-g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus like the one shown in Figure 6.5. Calculate the expansion work done against a constant external...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Shown here is a list published by Electronics Weekly.com of the top five semiconductor companies in the United States by revenue ($ billions). a. Construct a bar chart to display these data. b....
-
Do you think that standard costs are used only in making products like wheel bearings and hamburgers? Think again. Standards influence virtually every aspect of our lives. For example, the next time...
-
All other things being equal, species that inhabit cold climates tend to be larger than related species that inhabit hot climates. For instance, the Alaskan hare is the largest North American hare,...
-
Halley Enterprises bonds currently sell for $975. They have a 7-year maturity, an annual coupon of $90, and a par value of $1,000. What is their yield to maturity? AppendixLO1
-
Pat Sharpe enjoys listening to all types of music and owns countless CDs. Over the years, Pat has gained a local reputation for knowledge of music from classical to rap and the ability to put...
-
Note: oNLY Question 2 : how create graphic with excel Yellowstone Company began operations on January 1 to produce a single product. It used an absorption costing system with a planned production...
-
When the stems of cut flowers are held in concentrated NaCl(aq), the flowers wilt. In a similar solution a fresh cucumber shrivels up (becomes pickled). Explain the basis of these phenomena.
-
A 0.72 g sample of polyvinyl chloride (PVC) is dissolved in 250.0 mL of a suitable solvent at 25 C. The solution has an osmotic pressure of 1.67 mmHg. What is the molar mass of the PVC?
-
Determine the power supplied to the elements shown. 2 4 1 4 2 4
-
Scenario that Requires Conflict Resolution Provides a thorough and detailed summary outlining a workplace situation that requires conflict resolution including the setting, the parties involved and...
-
Select an industry that you are interested in, that is in your pathway, and a US company. Do an internet search with the term "sustainability report" and your company name. Once you find a company...
-
You are planning to buy a house in Toronto that has a price of $1,200,000. One of the local banks has offered you a mortgage at a quoted rate of 5% per year. Interest will be compounded semiannually....
-
Identify two significant concepts you have learned from each course You must have at least 6 courses with 12 total concepts. Identify the course in a heading, describe each concept, and explain why...
-
Use Trigonometric Identities to write each expression in terms of a single trigonometric identity or a constant. 1. cot sin 2. 1-sin sin20 1.) 2.) 3. sin 0 sec 0 cos 8 csc 8 cote 3.) Simplify the...
-
The circuit shown in Fig. 14.98 has the impedance Find: (a) The values of R, L, C, and G (b) The element values that will raise the resonant frequency by a factor of 10 3 by frequency scaling Figure...
-
On average there are four traffic accidents in a city during one hour of rush-hour traffic. Use the Poisson distribution to calculate the probability that in one such hour there arc (a) No accidents...
-
Describe some of the ways that the choice of accounting technique can temporarily depress or inflate earnings.
-
How would rapid inflation affect the accuracy and relevance of a manufacturing companys balance sheet and income statement? Does your answer depend on how much debt the company has issued?
-
In 1970 United Airlines bought four new jumbos for $21.8 million each. These planes were written down straight-line over 16 years to a residual value of $0.2 million each. However, they could have...
-
You have just sold a cemetery plot. As part of the sale, you have agreed to maintain the grass and flowers in perpetuity. You expect maintenance costs to be $62 due at the end of each year, forever....
-
Finance and management topics Which case will have more money at the end of 40 years? Case.1 Saving $1000 per year for 10 years starting in year 1 Case.2 Saving $1000 per year for 30 years starting...
-
If you calculate the payback period for your project as 4 years and your company has a cutoff payback period of 3 years, then your project meets your company's payback period requirement and will not...

Study smarter with the SolutionInn App


