We can use the heat liberated by a neutralization reaction as a means of establishing the stoichiometry
Question:
We can use the heat liberated by a neutralization reaction as a means of establishing the stoichiometry of the reaction. The data in the table are for the reaction of 1.00 M NaOH with 1.00 M citric acid, C6H8O7, in a total solution volume of 60.0 mL.
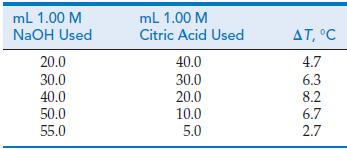
(a) Plot ΔT versus mL 1.00 M NaOH, and identify the exact stoichiometric proportions of NaOH and citric acid at the equivalence point of the neutralization reaction.
(b) Why is the temperature change in the neutralization greatest when the reactants are in their exact stoichiometric proportions? That is, why not use an excess of one of the reactants to ensure that the neutralization has gone to completion to achieve the maximum temperature increase?
(c) Rewrite the formula of citric acid to reflect more precisely its acidic properties. Then write a balanced net ionic equation for the neutralization reaction.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




