What are the coordination number and oxidation state of Co in the complex ion [CoCl(NO 2 )(NH
Question:
What are the coordination number and oxidation state of Co in the complex ion [CoCl(NO2)(NH3)4]+?
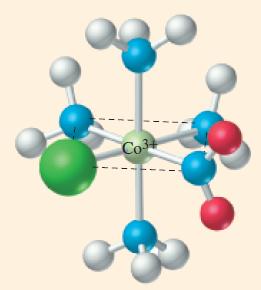
Transcribed Image Text:
Co34
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Analyze In determining the oxidation state of the metal ion in a complex it is important to recogniz...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
One mole of methane gas, with a temperature-independent heat capacity of 44//(mol - K), is held within a piston/cylinder device, at an initial condition of 600K and 5 MPa. It undergoes the following...
-
(A) What are the coordination number and oxidation state of nickel in the ion [Ni(CN) 4 I] 3 ? (B) Write the formula of a complex with cyanide ion ligands, an iron ion with an oxidation state of +3,...
-
What are the coordination number and the oxidation state of the central metal ion in each of the following complexes? Name each complex. (a) [Co(NH 3 ) 6 ] 2+ (b) [AlF 6 ] 3 (c) [Cu(CN) 4 ] 2 (d)...
-
Derek and Wes take a photograph of a lake at the same angle and the same time of day. Derek's picture comes out with crisp edges around objects in the photo. Wes's picture seems to blur the outlines...
-
Refer to the data in PE 9-3. Assume the company borrowed $20,000 of the purchase price from a bank. Make the necessary journal entry to record this transaction. Data from PE 9-3 K. Marie Company used...
-
CSM Corporation has a bond issue outstanding at the end of 2015. The bond has 15 years remaining to maturity and carries a coupon interest rate of 6%. Interest on the bond is compounded on a...
-
Determine whether the data are qualitative or quantitative. (a) A list of debit card pin numbers (b) The final scores on a video game
-
1. Do Problem 5- 25 using the FIFO method of process costing. Explain any difference between the cost per equivalent unit in the assembly department under the weighted- average method and the FIFO...
-
If you purchase a 5-year, zero-coupon bond for $600, how much could it be sold for 3 years later if interest rates have remained stable? $815.19 $1000 $985.23 $1,100
-
A complex of Al(III) can be formulated as AlCl 3 3 H 2 O. The coordination number is not known but is expected to be 4 or 6. Describe how Werners methods, that is, reaction with AgNO 3 (aq) or...
-
Absorbance is a measure of the proportion of monochromatic (single-color) light that is absorbed as the light passes through a solution. An absorption spectrum is a graph of absorbance as a function...
-
Flow to calculate and interpret margin and turnover using the DuPont model.
-
The composition of moist air is given on a molar basis to be 78 percent N2, 20 percent O2, and 2 percent water vapor. Determine the mass fractions of the constituents of air. Use the table containing...
-
1. Consider the LFSR with so = 1, 8 = 1, S2 = 1, 83 = 1, 84 = 0, and Sn Sn-2 Sn-3+ Sn-5. Find the next 15 terms in this LFSR. What is the period of this LFSR? 2. Suppose you learn that a Hill cipher...
-
Assume that you are thinking of a new acquisition campaign for SEDO, assuming that you want to convert people who are already engaged. Develop a big idea (in the communication) that you can use in...
-
You have a backend Amazon EC2 instance providing a web service to your web server instances. Your web servers are in a public subnet. You would like to block inbound requests from the internet to...
-
Consider the following task set. Task C T|D T1 20 50 40 T2 10 40 30 T3 5 20 15 a) Verify whether the task set is schedulable under DM using the processor utilization-based ap- proach. b) Verify...
-
For the field in Example 17.11, find each of the following: (a) [x + 2] [2.x + 2] + [x + 1] (b) [2x + l]2[x + 2] (c) (22)-1 = [2x + 2]-1
-
Could the owner of a business prepare a statement of financial position on 9 December or 23 June or today?
-
Using the data presented in BE13-4 for Rosalez Company, perform vertical analysis.
-
Net income was $500,000 in 2010, $485,000 in 2011, and $518,400 in 2012. What is the percentage of change from (a) 2010 to 2011, and (b) From 2011 to 2012? Is the change an increase or a decrease?
-
If Carolina Company had net income of $382,800 in 2012 and it experienced a 16% increase in net income over 2011, what was its 2011 net income?
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


