What volume of 18.5 C water must be added, together with a 1.23 kg piece of iron
Question:
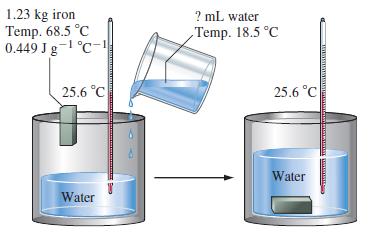
What volume of 18.5 °C water must be added, together with a 1.23 kg piece of iron at 68.5 °C, so that the temperature of the water in the insulated container shown in the figure remains constant at 25.6 °C?
Transcribed Image Text:
1.23 kg iron Temp. 68.5°C 0.449 Jg-1 °C-1 25.6 °C Water BASAARABALA ? mL water Temp. 18.5 °C 25.6 °C Water www MELATO TRATARE
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Q mcT where Q is the amount of heat transferred in Joules m is the mass of the substance in kilog...View the full answer

Answered By

Asd fgh
sadasmdna,smdna,smdna,msdn,masdn,masnd,masnd,m asd.as,dmas,dma.,sd as.dmas.,dma.,s ma.,sdm.,as mda.,smd.,asmd.,asmd.,asmd.,asm
5.00+
1+ Reviews
15+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
For the following exercises, graph the points and find a possible formula for the trigonometric values in the given table. 0 1 1 6 2 11 3 6 4 1 5 6
-
What volume of 18.5 C Water must be added together , with a 1.23 kg piece of iron at 68.5 C , so that the temprature of the water in the insulated container such as the temprature remains constant at...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
State with reasons, whether the following statements are true or false : (i) Overhauling expenses for the engine of motor car to get better fuel efficiency is revenue expenditure. (ii) Depreciation...
-
Assume that you are the managerial accountant at Infostore, a manufacturer of hard drives, CDs, and diskettes. Its reporting year-end is December 31. The chief financial officer is concerned about...
-
Compute the zero-coupon bond prices from the following forward rates. Times to Maturity T Today's forward Rates f(O,T) f(0,2) f(0,3) f(0,4) 0.02 0.03 0.04 0.05 3 4
-
BASICS OF CAPITAL BUDGETING You recently went to work for Allied Components Company, a supplier of auto repair parts used in the after-market with products from Daimler AG, Ford, Toyota, and other...
-
A plant manager is interested in developing a quality-control pro-gram for an assembly line that produces light bulbs. To do so, the manager considers the quality of the products that come from the...
-
please look at this one this is the wrong answer. don't repeat this answer! Waterways Corporation uses very stringent standard costs in evaluating its manufacturing efficiency. These standards are...
-
Lakeland-Bering Aircraft Company is preparing a contract proposal to submit to the defense department for a new military aircraft, the X-300J jet fighter. Part of the proposal is a development and...
-
A British thermal unit (Btu) is defined as the quantity of heat required to change the temperature of 1 lb of water by 1 F. Assume the specific heat capacity of water to be independent of...
-
Calculate the enthalpy of combustion for lactic acid by using the data in Table 7.2 and the standard enthalpy of formation for lactic acid [CH 3 CH(OH)COOH(s)]: f H = -694.0 kJ/mol. Table 7.2 TABLE...
-
A new gaselectric hybrid car has recently hit the market. The distance traveled on 1 gallon of fuel is normally distributed with a mean of 65 miles and a standard deviation of 4 miles. Find the...
-
What is XYZ Corp.'s net cash flow XYZ Corp. (for 2020) Revenue $5,000,000 Wages: $1,000,000 D&A: $1,000,000 Property, Plant & Equipment investment: $1,500,000 Tax Rate: 35% NOWC (2020): $750,000 NOWC...
-
Tower x (m) y (m) UU3 -118.1 -15.6 OU1 -85.3 -15.9 Sensor heights (m) 3.19, 4.16, 5.04, 7.24, 9.84 1.5, 3.0, 5.46, 9.86, 15.65 OU2 -90.0 -8.3 1.5, 2.96, 5.97, 9.91, 15.08 ASU -22.8 -8.6 5.0 UUT -13.3...
-
For each of the matrices determine the value(s) of c for which the given matrix is not invertible. [4 25. 26. 3 5 } ] 6 27. 28. 2 c+4 C -8 c-6]
-
Spitfire Company makes and sells three products: A, B, and C. The following data relate to these products: A B Demand in units Selling price per unit 110 100 90 $180 $210 $195 Raw material costs per...
-
NCF & Partners (NCF) is a firm of CPAslocated in Whitby that has been in business for 20 years. NCF's revenue has declined steadily over the past few years. The partners are looking for ways...
-
Following data have been obtained for machining AA390 Aluminum, a Si-Al alloy. Compute the K and n values for the Taylor tool life equation. How do these n values compare to the typical values?...
-
Which internal control principle is especially diffi cult for small organizations to implement? Why?
-
Acquisition Costs of Trucks Shabbona Corporation operates a retail computer store. To improve delivery services to customers, the company purchases four new trucks on April 1, 2010. The terms of...
-
Purchase and Self-Constructed Cost of Assets Dane Co. both purchases and constructs various equipment it uses in its operations. The following items for two different types of equipment were recorded...
-
Treatment of Various Costs Allegro Supply Company, a newly formed corporation, incurred the following expenditures related to Land, to Buildings, and to Machinery and Equipment. Determine the amounts...
-
he following disclosure note appeared in the December 31, 2016, annual report of the Intel Corporation. Note 5: Cash and Investments (partial) Available-for-sale investments as of December 31, 2016,...
-
Velk Sarage reports the following information: Direct tabor rate Non-materiats-related overhead Materials-related overhead Target profit arain (on both conversion and durect asteriats) 5 the per out...
-
I need help here, thumbs are ready !! HW 4 extra For the flowing "Scan Design" problem: a). Fill in the Values for the three Flip flops (with reference to the circuit on the back of this page), probe...

Study smarter with the SolutionInn App


