When molten sulfur reacts with chlorine gas, a vilesmelling orange liquid forms. When analyzed, the liquid compound
Question:
When molten sulfur reacts with chlorine gas, a vilesmelling orange liquid forms. When analyzed, the liquid compound has the empirical formula SCl. Several possible Lewis structures are shown below. Criticize these structures and choose the best one.
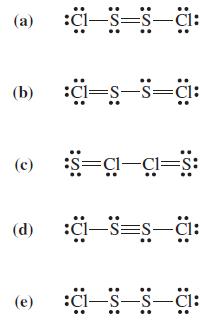
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





