Which of the following names is most appropriate for the molecule with the structure shown below? (a)
Question:
Which of the following names is most appropriate for the molecule with the structure shown below?
(a) Butyl alcohol;
(b) Butan-2 ol;
(c) Butan-1-ol;
(d) Isopentyl alcohol.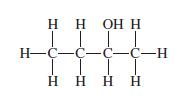
Transcribed Image Text:
НННН ТТТТ H-C-с-с-с-н | | Н НО Н Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
The correct answer is B Butan2ol accurate...View the full answer

Answered By

Jinah Patricia Padilla
Had an experience as an external auditor in Ernst & Young Philippines and currently a Corporate Accountant in a consultancy company providing manpower to a 5-star hotel in Makati, Philippines, Makati Diamond Residences
5.00+
120+ Reviews
150+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Which of the following names is most appropriate for the molecule CH 3 (CH 2 ) 2 COOH? (a) Dimethyleneacetic acid; (b) Propanoic acid; (c) Butanoic acid; (d) Oxobutylalcohol.
-
Which of the following pairs of compounds has a. The higher boiling point: 1-bromopentane or 1-bromohexane? b. The higher boiling point: pentyl chloride or isopentyl chloride? c. The greater...
-
1. Hannah is applying for a life policy on her girlfriend Sarahs life. The policy is $500,000 and carries a large premium. Hannah is the main earner, so she is concerned about not being able to pay...
-
What is a reporting entity?
-
The following data relate to a $2,000,000, 8% bond issue for a selected semiannual interest period: Bond carrying amount at beginning of period ...... $2,125,000 Interest paid during period...
-
Jane Doe earns $ 30,000 per year and has applied for an $ 80,000, 30-year mortgage at 8 percent interest, paid monthly. Property taxes on the house are expected to be $ 1,200 per year. If her bank...
-
NPV AND IRR A store has 5 years remaining on its lease in a mall. Rent is $2,000 per month, 60 payments remain, and the next payment is due in 1 month. The malls owner plans to sell the property in a...
-
1. Create a decision table that describes the movement of inventory. 2. Draw a decision tree that describes the merchandise inventory management process. 3. Name four attributes that you can use to...
-
The Activity-Based costs assigned to cost objects are based on what? Multiple Choice Activity cost pools. Actual levels of spending. Actual levels of capacity usage. Planned levels of spending and...
-
An 8.129 g sample of MgSO 4 x H 2 O is heated until all the water of hydration is driven off. The resulting anhydrous compound, MgSO 4 weighs 3.967 g. What is the formula of the hydrate?
-
A certain hydrate is found to have the composition 20.3% Cu, 8.95% Si, 36.3% F, and 34.5% H 2 O by mass. What is the empirical formula of this hydrate?
-
The following supply and demand schedules describe a hypothetical Canadian market for potash. Price ($ Quantity Supplied Quantity Demanded (million tonnes) per tonne) (million tonnes) 12.5 280 8.5...
-
in a thermodynamics, a phase means what?
-
Give me a 3 python codes in (Discrete Mathematics course) for : 1-basic algorithm 2- the growth of functions 3- Complexity of Algorithms And explain how its works with examples.
-
Using the perpetual inventory system, calculate the ending inventory and Cost of Goods Sold under each of the following methods. Beginning Inventory 10 units @ $1 Purchases January 5 January 20 20...
-
the manager of wongs food express estimates operating costs for the year will total $300,000 for fixed costs. 28. find the sales dollars required with a contribution margin ratio of 40 percent to...
-
Tara Williams and Tilly North had been yoga buddies for almost a decade. Yoga was an escape from the daily stresses of being working parents for both of them. One thing Tara and Tilly always talked...
-
Give the products of the following reactions. Show all stereoisomers that are formed. a. b. c. d. 1. (CH32CuLl 2. H30 1. CH3MgBr 2. H30 CH3 catalytic Ht CCH2CH3 + 1. LIAIH4 CH CH,CCH CH,CH2CH 2. HO
-
Clark, PA, has been engaged to perform the audit of Kent Ltd.s financial statements for the current year. Clark is about to commence auditing Kents employee pension expense. Her preliminary enquiries...
-
Grunewald Industries sells on terms of 2/10, net 40. Gross sales last year were $4,562,500, and accounts receivable averaged $437,500. Half of Grunewalds customers paid on the 10th day and took...
-
The D.J. Masson Corporation needs to raise $500,000 for 1 year to supply working capital to a new store. Masson buys from its suppliers on terms of 3/10, net 90, and it currently pays on the 10th day...
-
The Zocco Corporation has an inventory conversion period of 75 days, a receivables collection period of 38 days, and a payables deferral period of 30 days. a. What is the length of the firms cash...
-
On January 1, 2017, Metlock Inc. borrowed and received $400,000 from a major customer, Bridgeport Corp. The debt is evidenced by a zero-interest-bearing note due in 4 years. Metlock, as consideration...
-
Denny Corporation is considering replacing a technologically obsolete machine with a new state-of-the-art numerically controlled machine. The new machine would cost $290,000 and have a tenericals...
-
Prepare journal entries for the following transactions from Restaurant Depot Nov. 8 Customer Miles Shandy purchased 230 pans at $39 per par, costing Restaurant Depot $26 per pan. Terms of the sale...

Study smarter with the SolutionInn App


