Which of the following reactions are likely to go to completion or very nearly so? (a) (b)
Question:
Which of the following reactions are likely to go to completion or very nearly so?
(a)![]()
(b)
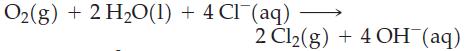
(c)
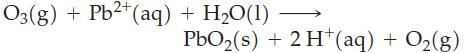
(d)
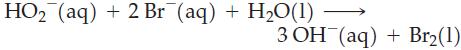
Transcribed Image Text:
H₂O₂(aq) + 21¯(aq) + 2 H¹(aq) I2(s) + 2 H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
To determine which reactions are likely to go to completion or very nearly so we can analyze the rea...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Homework-2 DECISION ANALYSIS PROBLEMS Problem-1: Kenneth Brown is the principal owner of Brown Oil, Inc. After quitting his university teaching job, Ken has been able to increase his annual salary by...
-
Project the 2 4-1 IV design in Example 8-1 into two replicates of a 2 2 design in the factors A and B. Analyze the data and thaw conclusions. Example 8-1: Consider the filtration rate experiment in...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
CPA firm brings in a yoga instructor during the tax busy season to help relieve stress of the employees. Which is true about the CPA firm's ability to take a deduction for the yoga instructor's...
-
Refer to the information in E 8-24. If those holding stock options can purchase a share of stock for $48 and the market value of a share of stock on January 1, 2012, is $50, how can the option to...
-
What is the velocity of an electron that has a momentum of 3.04 10-21 kgm/s? Note that you must calculate the velocity to at least four digits to see the difference from c?
-
20-1. Qu son las ventas personales ?
-
In March 2018, the management team of Londonderry Air (LA) met to discuss a proposal to purchase five shorthaul aircraft at a total cost of $25 million. There was general enthusiasm for the...
-
You have just been hired by FAB Corporation, the manufacturer of a revolutionary new garage door opening device. The president has asked that you review the company's costing system and "do what you...
-
Use Lewis structures and other information to explain the observation that (a) H 2 S is a gas at room temperature, whereas H 2 O is a liquid. (b) O 3 is diamagnetic.
-
In water, O 2 (aq) is a strong base. If 100.0 mg of K 2 O(s) is dissolved in 1.25 L of aqueous solution, what will be the pH of the solution?
-
Amsterdam Ltd and Berlin Ltd are both wholesalers serving broadly the same market, but they seem to take a different approach to it according to the following information: Required: Describe what...
-
Locate a scholarly article relevant to how to present your financial plan for opening a Roller Skating Rink (from your draft business plan) to a lending institution--and describe your strategy for...
-
How would you expect seasonal fluctuations in demand to affect a rental company's decisions about pricing rented products such as wedding dresses or convertible cars? In terms of pricing principles,...
-
Do we drive technology, or does technology drive us? If technology drives us, what are the risks? The other side of the coin would be that we are able to stay ahead of technological transformations....
-
How do you explain the differences between the two analyses and what are the implications of using the BCG matrix in practice?
-
How do leadership styles, such as transformational leadership, shared leadership, and servant leadership, impact team dynamics, member motivation, and overall team effectiveness ?
-
Complete the proofs of Theorems 14.15 and 14.16.
-
Consider the combustion of methanol below. If 64 grams of methanol reacts with 160 grams of oxygen, what is the CHANGE in volume at STP. 2CH3OH(g) + 3O2(g) 2CO2(g) + 4H2O(1) The volume decreases by...
-
What principles of internal control apply to most businesses?
-
Graham Moran is reviewing the principle of segregation of duties. What are the two common applications of this principle?
-
How do documentation procedures contribute to good internal control?
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


