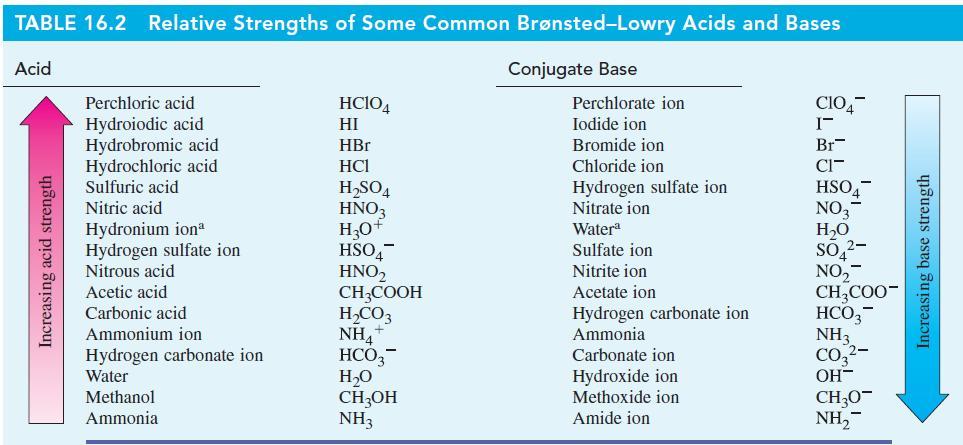
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the
Question:
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the following acid–base reactions.
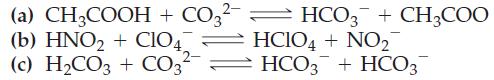
Table 16.2
Transcribed Image Text:
(a) CH3COOH + CO3²- = HCO3 + CH3COO (b) HNO₂ + ClO4 2- HCIO4 + NO₂ HCO3 + HCO3 (c) H₂CO3 + CO3²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To predict the direction favored in acidbase reactions you can consider the strengths of the acids a...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
62+ Reviews
349+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the following acidbase reactions. Table 16.2 (a) NH4+ + OH = HO + NH3 (b) HSO4 + NO3 HNO3 + SO4- (c) CH3OH +...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
With the aid of a periodic table (not Figure 9.15), arrange the following in order of increasing electronegativity: a. Sr, Ca, Rb b. Ca, Ge, Ga c. Se, As, Sb
-
Phoenix Corp. faltered in the recent recession but is recovering. Free cash flow has grown rapidly. Forecasts made in 2016 are as follows. Phoenix's recovery will be complete by 2021, and there will...
-
What is a revolving credit agreement? How does this arrangement differ from the line-of-credit agreement? What is a commitment fee?
-
Martin's Service Station is considering entering the snowplowing business for the coming winter season. Martin can purchase either a snowplow blade attachment for the station's pick-up truck or a new...
-
4. Companies in highly competitive industries often see a number of consecutive restructuring charges. In these cases, should restructuring be treated as operating or nonoperating? From a valuation...
-
Assume the same facts as for Question 1 above. The fair value of the investment in Company E is $220,000 on December 31, 2011. Answer the following questions assuming the investment is recorded using...
-
The throughput time was: 41.4 hours 34.1 hours 14.2 hours 7.3 hours
-
(A) What is the pH of 0.015 M CH 2 FCOOH(aq)? (B) Piperidine is a base found in small amounts in black pepper. What is the pH of 315 mL of an aqueous solution containing 114 mg piperidine? CHFCOOH +...
-
Show by calculation that the pH of 0.100 M CH 3 COOH should be about the value shown on the pH meter in Figure 16-6; that is, pH 2.8. Figure 16-6 1.20 20 2.80. 19
-
Which candidate is the winner by the Borda count method? Use the table below. Number of Ballots 100 80 110 105 55 Candidate A 1 1 4 4 2 Candidate B 2 2 2 3 1 Candidate C 4 4. 1 1 4 Candidate D 3 3 3...
-
Consider a rigid body B with center of mass point B*. A set of coordinate axes is chosen centered at B* and defined by mutually orthogonal unit vectors 61, 62, 63 which are fixed in B. The rigid body...
-
Let A be the matrix 1 0 2 4 1 -6 = 7-4 7 -5 3 (a) (2 points) What must a and b be in order to define the linear transformation T: RR by T(x) = Ax. (b) (3 points) What is the image of the vector 2] 1...
-
write a title Understanding the roots of modern educational practices can provide valuable insights into their effectiveness and potential for improvement. One such root influencing contemporary...
-
State the limit for each of the following using the graph. -6. -5 + -3- 3 -2 -2 0 2 -2- w. 3 4
-
Great Eastern Credit Union (GECU) has two operating departments (Branches and Electronic) and three service departments (Processing, Administration, and Maintenance). During July, the following costs...
-
Hernandez Company issued $380,000, 7%, 10-year bonds on January 1, 2017, for $407,968. This price resulted in an effective-interest rate of 6% on the bonds. Interest is payable annually on January 1....
-
In the figure, two loudspeakers, separated by a distance of d1 = 2.63 m, are in phase. Assume the amplitudes of the sound from the speakers are approximately the same at the position of a listener,...
-
Catherine Simpkins owns and operates Speedy Print Co. During February, Speedy Print Co. incurred the following costs in acquiring two printing presses. One printing press was new, and the other was...
-
Bridger Ski Co. has developed a tract of land into a ski resort. The company has cut the trees, cleared and graded the land and hills, and constructed ski lifts. (a) Should the tree cutting, land...
-
Fastball Delivery Company acquired an adjacent lot to construct a new warehouse, paying $30,000 and giving a short-term note for $270,000. Legal fees paid were $1,425, delinquent taxes assumed were...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


