With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the
Question:
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the following acid–base reactions.
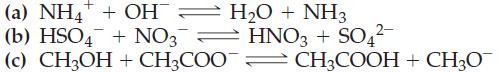
Table 16.2
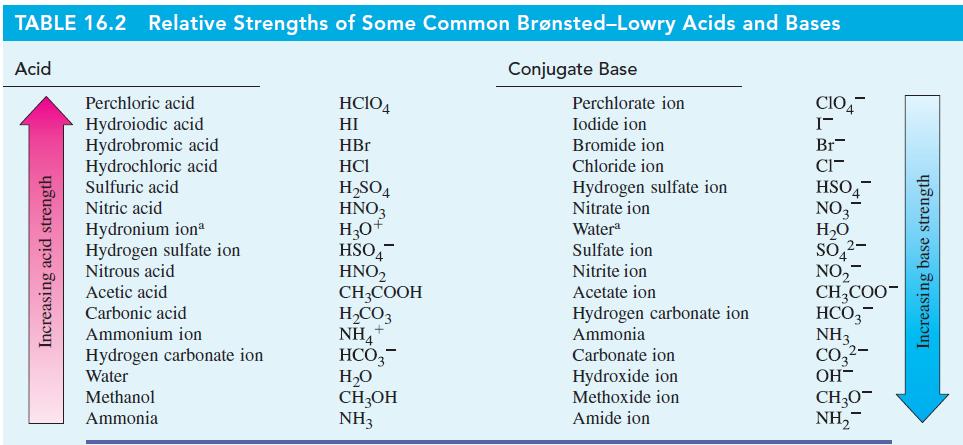
Transcribed Image Text:
(a) NH4+ + OH = H₂O + NH3 (b) HSO4 + NO3 HNO3 + SO4²- (c) CH3OH + CH3COOCH3COOH + CH3O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the following acidbase reactions. Table 16.2 (a) CH3COOH + CO3- = HCO3 + CH3COO (b) HNO + ClO4 2- HCIO4 + NO...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
With the aid of a periodic table (not Figure 9.15), arrange the following in order of increasing electronegativity: a. Sr, Ca, Rb b. Ca, Ge, Ga c. Se, As, Sb
-
The CFO of the Jordan Microscope Corporation intentionally misclassified a downstream transportation expense in the amount of $575,000 as a product cost in an accounting period when the company made...
-
How is the prime rate of interest relevant to the cost of short-term bank borrowing? What is a floating-rate loan?
-
In what circumstances might a market-pull approach or a technology-push approach to new-product design be the best approach?
-
3. Many financial analysts estimate the value of operating leases by discounting rental payments provided in the annual report at the cost of debt. Is this method likely to overestimate or...
-
Last year, only four of 32 professional sports teams in a new league turned a nominal accounting profit. Betty purchased such a team this year. Her taxable loss was determined to be $ 950,000. Can...
-
7. When an investor holds a stock that has lost nearly all of its value, the investor will often times sell the stock to her stock broker for the total sum of $1. Why do they do that? Legally, what...
-
The equation representing the neutralization of acetic acid, CH 3 COOH, by a base B is CH 3 COOH(aq) + B(aq) CH 3 COO - (aq) + BH + (aq). Of the bases listed in Table 16.4, which would be effective...
-
(A) Substituting halogen atoms for hydrogen atoms bound to carbon increases the strength of carboxylic acids. Show that the pH of 0.100 M CH 2 FCOOH, fluoroacetic acid, is lower than that calculated...
-
Consider two groups of students: B 1 , students who received high scores on tests, and B 2 , students who received low scores on tests. In group B 1 , 60% study more than 25 hours per week, and in...
-
Winston Electronics reported the following information at its annual meetings. The company had cash and marketable securities worth $1,235,740, accounts payables worth $4,160,391, inventory of...
-
Hooray Company has been manufacturing 12,000 units of Part A which is used to manufacture one of its products. At this level of production, the cost per unit is as follows: Direct materials P 4.80...
-
At the beginning of the period, the Grinding Department budgeted direct labor of $171,200 and property tax of $57,000 for 10,700 hours of production. The department actually completed 12,800 hours of...
-
The following information is available for Shamrock Corporation for the year ended December 31, 2025. Beginning cash balance $ 58,500 Accounts payable decrease 4,810 Depreciation expense 210,600...
-
In today's stock market, compounding is the key to making money in the future for one's investments. However, with decentralized currency growing rapidly (Crypto), how can one rely on TVM for FV...
-
On May 15, Wild Quest Clothiers borrowed some money on a 4-month note to provide cash during the slow season of the year. The interest rate on the note was 8%. At the time the note was due, the...
-
1. Following are information about Alhadaf Co. Cost incurred Inventory Purchases Sales Adverting expense Salary Expense Depreciation Beginning Inventory Ending Inventory Amount 118,000 350.000 90,000...
-
A storage tank acquired at the beginning of the fiscal year at a cost of $172,000 has an estimated residual value of $20,000 and an estimated useful life of eight years. Determine the following: (a)...
-
Sandblasting equipment acquired at a cost of $85,000 has an estimated residual value of $5,000 and an estimated useful life of 10 years. It was placed in service on October 1 of the current fiscal...
-
A building with a cost of $1,050,000 has an estimated residual value of $420,000, has an estimated useful life of 36 years, and is depreciated by the straight-line method. (a) What is the amount of...
-
Construction of consumer price index number for the given goods and services. Item Weight in % Base period price Current period price Food 35 150 145 Fuel 10 25 23 Cloth 20 75 65 Rent 15 30 30 Misc....
-
Gammaro Corporation has found that 80% of its sales in any given month are credit sales, while the remainder are cash sales of the credit sales, Gammaro Corporation has experienced the following...
-
Swifty Company estimates that 2022 sales will be $43,200 in quarter 1,$51,840 in quarter 2 , and $62,640 in quarter 3 , Cost of goods sold is 50% of sales. Management desires to have ending...

Study smarter with the SolutionInn App


