Without performing detailed calculations, determine which decomposition yields the most O 2 (g) (a) Per mole and
Question:
Without performing detailed calculations, determine which decomposition yields the most O2(g)
(a) Per mole and
(b) Per gram of substance.
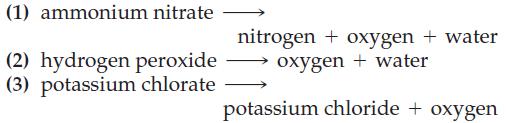
Transcribed Image Text:
(1) ammonium nitrate (2) hydrogen peroxide (3) potassium chlorate nitrogen + oxygen + water oxygen + water potassium chloride + oxygen
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Without detailed calculations its known that potassium chlorate KClO3 decom...View the full answer

Answered By

Jinah Patricia Padilla
Had an experience as an external auditor in Ernst & Young Philippines and currently a Corporate Accountant in a consultancy company providing manpower to a 5-star hotel in Makati, Philippines, Makati Diamond Residences
5.00+
120+ Reviews
150+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Calculate three annual totals of Liquidity, Asset Mgmt., Debt Mgmt. and Profitability ratios Ratios to calculate (per year): Liquidity - current ratio, quick ratio Asset Management - inventory...
-
The heating capacity of a calorimeter is known to be 4 kJ/oC, with negligible uncertainty. The number of dietary calories (kiloCalories) per gram of a substance is given by C = cH(T )/m, where C is...
-
Do you agree or not? Specialization of labor and better use of capital goods can initially generate increasing marginal output (returns) for a firm in the production of a good.
-
Which one of the following statements correctly describes income tax expense? a. The amount of cash paid for income taxes during the year b. The amount of income tax owed as of the end of the year c....
-
Is it harder to transform the organization or the human resource? Explain.
-
1 Jane Dawson es una nueva representante de ventas de la empresa de corretaje Charles Schwab. En su bsqueda de clientes, Jane compr una lista de correo de suscriptores de The Wall Street Journal y...
-
Dr. Smith learned that one sorority on campus had purchased several Macintosh computers and another sorority had purchased several Windows-based computers. Dr. Smith was interested in whether the...
-
Activity Casino Assembly Inspecting Budgeted Activity Cost Activity Base $500,000 Machine hours 75.000 Direct labor hours 30.000 Number of inspections 18.750 Number of setups 14,000 Number of loads...
-
Without performing detailed calculations, determine which of the following compounds has the greatest percent oxygen by mass: dinitrogen tetroxide, aluminum oxide, tetraphosphorus hexoxide, or carbon...
-
Each of the following compounds decomposes to produce O 2 (g) when heated: (a) HgO(s); (b) KClO 4 (s). Write plausible equations for these reactions.
-
a. Express 12 sin x + 5 cos x in the form R sin (x + ), where R and are constants, R > 0 and 0 < < 90. Round to 1 decimal place. A runners speed, v in m/s, in an endurance race can be modelled by...
-
How do transnational organizations and agreements influence national sovereignty and political autonomy ?
-
How do individuals reconcile the tension between rational deliberation and emotional impulses when making consequential decisions amidst volatile environments, and to what extent does the phenomenon...
-
Watch the video clip below; https://www.youtube.com/watch?v=sE6Ox3ikCMU 1. Do you think that 'Rick and Morty' was a good choice? Justify your answer. 2. Do you think that this campaign will work for...
-
by the hypothesis that we want to do descriptive method, and quantative research in Tim hortons company, the question is A convincing closing statement, including that you'll develop your research...
-
Bottom of Form Why do you think ethics is important in healthcare management? What do you see as the biggest risks and temptations? How is your INTEGRITY a core principle in your professional ethical...
-
Let a, b, m, n Z with m, n > 0. Prove that if a = b (mod n) and m , then a = b (mod m).
-
How has the too-big-to-fail policy been limited in the FDICIA legislation? How might limiting the too-big-to-fail policy help reduce the risk of a future banking crisis?
-
The following information is available for Eckman Company for the month of January: expected cash receipts $59,000; expected cash disbursements $67,000; and cash balance on January 1, $12,000....
-
On March 20, Palaszs petty cash fund of $100 is replenished when the fund contains $19 in cash and receipts for postage $40, supplies $26, and travel expense $15. Prepare the journal entry to record...
-
Identify which control activity is violated in each of the following situations, and explain how the situation creates an opportunity for fraud or inappropriate accounting practices. 1. Once a month,...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 0 2 0 2 1 $ 6 4...
-
The adjusted trial balance for Tybalt Construction on December 3 1 of the current year follows. TYBALT CONSTRUCTION Adjusted Trial Balance December 3 1 Number Account Title Debit Credit 1 0 1 Cash $...
-
( US$ millions ) 1 2 / 3 1 / 2 0 1 4 1 2 / 3 1 / 2 0 1 3 1 2 / 3 1 / 2 0 1 2 1 2 / 3 1 / 2 0 1 1 Net income $ 1 4 , 4 3 1 $ 1 2 , 8 5 5 $ 1 0 , 7 7 3 $ 9 , 7 7 2 Depreciation 3 , 5 4 4 2 , 7 0 9 1 ,...

Study smarter with the SolutionInn App


