Write a balanced chemical equation for the reaction depicted below. +
Question:
Write a balanced chemical equation for the reaction depicted below.
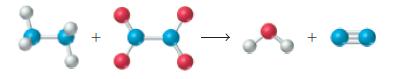
Transcribed Image Text:
+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
The balanced chemical equation for the reaction depicted in the image is 2 SiCl4 2 NH4Cl 2 SiF4 2 N...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced chemical equation for the reaction that occurs when (a) Calcium metal undergoes a combination reaction with O2(g) (b) Copper(II) hydroxide decomposes into copper(II) oxide and water...
-
Write a balanced chemical equation for the reaction that occurs when (a) Mg(s) reacts with Cl2(g) (b) Barium carbonate decomposes into barium oxide and carbon dioxide gas when heated (c) The...
-
Write a balanced chemical equation for the pentose phosphate pathway in the first two modes depicted in Figure 12.36, where (a) ribose-5-phosphate synthesis is maximized (b) NADPH production is...
-
A company is deciding whether to produce a new gadget at a plant located in a country close to consumers at a higher labor cost and shorter lead time or to outsource it to a country with a low labor...
-
Using the data for Geo-Metrics Corporation in Exercise 14-20, assume that as of December 31, 2008, the M-Labs Inc. stock had a market value of $25 per share and the Spectrum Corp. stock had a market...
-
Describe the three standard-setting organizations involved in writing auditing standards and the roles of each in establishing guidelines for auditors.
-
At September 30, the end of Beijing Companys third quarter, the following stockholders equity accounts are reported. In the fourth quarter, the following entries related to its equity are recorded:...
-
Discuss Nokias global strategy in terms of the five global product and communications strategies. What brand of cell phone do you own? If youre living in the United States, chances are it isnt a...
-
Current Attempt in Progress The following irformation is available for Sage Hill Company. Prepare the cost of goods manufisctured schedule for the month of April 2022. (Assume that all raw materials...
-
Phosphorus trichloride, PCl 3 is a commercially important compound used in the manufacture of pesticides, gasoline additives, and a number of other products. A ball-and-stick model of PCl 3 is shown...
-
Write balanced equations to represent: (a) The reaction of sulfur dioxide gas with oxygen gas to produce sulfur trioxide gas (one of the reactions involved in the industrial preparation of sulfuric...
-
Activity Cost Drivers and Cost Estimation Market Street Soup Company produces ten varieties of soup in large vats, several thousand gallons at a time. The soup is distributed to several categories of...
-
This case study is based on a fictional character on NBC's The Office. Michael is the central character of the series, serving as the Regional Manager of the Scranton branch of a paper distribution...
-
What is the significance of a balance sheet in understanding a firm's financial position? How do changes on the right side of the balance sheet (liabilities and equity) impact a company's financial...
-
A current event analysis where the article must focus on a management concepts). You will read the article and then provide an analysis of the subject matter discussed. The article should complement...
-
Given an exponential distribution with =20, what is the probability that the arrival time is a. less than X=0.2? b. greater than X = 0.2? c. between X=0.2 and X 0.3? d. less than X=0.2 or greater...
-
Choose at least two measures of employee attitudes. Discuss them and tell me about your discussion. Which group you believe are the most effective and efficient measures? Why? 2) Discuss turnover,...
-
The following reaction is known as the benzoin condensation. The reaction will not take place if sodium hydroxide is used instead of sodium cyanide. Propose a mechanism for the reaction. CH3OH benzoin
-
Using the information presented in Problem 13.4B, prepare a partial statement of cash flows for the current year, showing the computation of net cash flows from operating activities using the...
-
What is free cash flow? Why is it the most important measure of cash flow?
-
If you were starting a business, what tax considerations might cause you to prefer to set it up as a proprietorship or a partnership rather than as a corporation?
-
An investor recently purchased a corporate bond which yields 9 percent. The investor is in the 36 percent combined federal and state tax bracket. What is the bonds after-tax yield?
-
5. Volunteer Manufacturing was acquired by Trajectory Manufacturing. Trajectory was interested in expanding its product line to include Volunteer's most popular Volunteer provided Trajectory with the...
-
1. What is amount of cash inflow from operations? 2. What is amount of cash outflow from operations? 3. What is amount of loan balance at the end of January after loan repayments, if any? XYZ...
-
On January 1, 2020, Palka, Inc., acquired 70 percent of the outstanding shares of Sellinger Company for $1,277,500 in cash. The price paid was proportionate to Sellingers total fair value, although...

Study smarter with the SolutionInn App


