Write condensed structural formulas for phosphoric acid and phosphorous acid. Condensed structural formulas are discussed on page
Question:
Write condensed structural formulas for phosphoric acid and phosphorous acid. Condensed structural formulas are discussed on page 70.
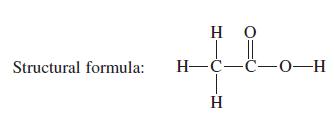
Transcribed Image Text:
Structural formula: HO || H-C-C-0-H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
H P OH HO P O...View the full answer

Answered By

ZIPPORAH KISIO LUNGI
I have worked on several other sites for more than five years, and I always handle clients work with due diligence and professionalism. Am versed with adequate experience in the fields mentioned above in which have delivered quality papers in research, thesis, essays, blog articles, and so forth.
I have gained extensive experience in assisting students to acquire top grades in biological, business and IT papers. Notwithstanding that, I have 7+ years of experience in corporate world software design and development.
5.00+
194+ Reviews
341+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced chemical equation using condensed structural formulas for (a) the formation of butyl propionate from the appropriate acid and alcohol, (b) the saponification (base hydrolysis) of...
-
Write a balanced chemical equation using condensed structural formulas for the saponification (base hydrolysis) of (a) methyl propionate, (b) phenyl acetate.
-
Write a balanced chemical equation using condensed structural formulas for the saponification (base hydrolysis) of (a) methyl propionate, (b) phenyl acetate. Discuss.
-
Which description does NOT fit in description of "issues" in the context of international standards? a. An unsettled matter. b. A vital matter. c. A change in the environment. d. A concern or...
-
When and how does a company record the amount owed to the government for income taxes for a given year?
-
Explain why the demand for labor is a derived demand using examples from your workplace. What are some historic examples of products that are no longer demanded and what was the impact on labor?
-
What is bias error in forecasting? What are some of the causes? LO.1
-
Gibralter Insurance Company uses a flexible overhead budget for its application-processing department. The firm offers five types of policies, with the following standard hours allowed for clerical...
-
You have been given the following return information for a mutual fund, the market index, and the risk-free rate. You also know that the return correlation between the fund and the market is 0.89....
-
Some sources of natural gas contain 8% He by volume. How many liters of such a natural gas must be processed at STP to produce 5.00 g of He?
-
In the solid phase, PCl 5 forms PCl 4 + and PCl 6 + However, PBr 5 forms PBr 4 + Br Suggest a reason for this difference in structure.
-
In World War I, the most awesome weapons of war were huge cannons mounted on railcars. Figure shows such a cannon, mounted so that it will project a shell at an angle of 30. With the car initially at...
-
You are expected to suggest several functional tactics and how these short-term activities are used to achieve short term objectives and establish a competitive advantage. Within the general...
-
Carbon dioxide and nitrogen experience equimolar counterdiffusion in a circular tube whose length and diameter are 1m and 50mm, respectively. The system is at a total pressure of 1 atm and a...
-
A licensee recently was placed on court - ordered probation. Does the licensee have to report this to the Board?
-
1. Technology and Operations What task does the operations function in a manufacturing organisation and in a service organisation perform? How does operations strategy contribute to make to corporate...
-
Do the Following current market analysis - geographic , psychographic and behavioral of Klean Kanteen THIS IS THE DETAILS AND DRAFTS OF PAPER. (THIS IS THE BASIS) Open the link;...
-
(a) Prove that for all n N, 10" = (-1)" (mod 11). (b) Consider the result for mod 9 in part (b) of Exercise 18. State and prove a comparable result for mod 11.
-
A business had revenues of $280,000 and operating expenses of $315,000. Did the business (a) Incur a net loss (b) Realize net income?
-
What is the LIFO reserve? What are the consequences of ignoring a large LIFO reserve when analyzing a company?
-
When perpetual inventory records are kept, the results under the FIFO and LIFO methods are the same as they would be in a periodic inventory system. Do you agree? Explain.
-
Nicholas Company discovers in 2012 that its ending inventory at December 31, 2011, was $5,000 understated. What effect will this error have on (a) 2011 net income, (b) 2012 net income, and (c) The...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


