Write equilibrium constant expressions, K c , for the reactions 2 NO2(g) Zn+ Zn+ (aq) + 2
Question:
Write equilibrium constant expressions, Kc, for the reactions
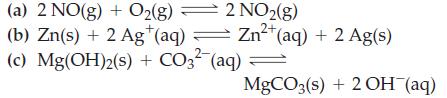
Transcribed Image Text:
2 NO2(g) Zn²+ Zn²+ (aq) + 2 Ag(s) 2+ (a) 2 NO(g) + O2(g) (b) Zn(s) + 2 Ag+ (aq) = (c) Mg(OH)2(s) + CO3²- (aq) = MgCO3(s) + 2OH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
To write the equilibrium constant expressions Kc for the given reaction...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
62+ Reviews
349+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The solution containing no added KNO3 for Figure 7-1 contains 5.0 mM Fe(NO3)3, 5.0 M NaSCN, and 15 mM HNO3. We will use Davies activity coefficients to find the concentrations of all species in the...
-
Write the expressions for Kc for the following reactions. In each case indicate whether the reaction is homogeneous or heterogeneous.
-
For the synthesis of ammonia the equilibrium constant Kc at 375°C is 1.2. Starting with [H2]0 = 0.76 M, [N2]0 = 0.60 M, and [NH3]0 = 0.48 M, which gases will have increased in concentration and...
-
Both of the following questions are essentially the same. Does the difference in wording seem as though it could affect the way that people respond? Are you in favor of the "Defense of Marriage...
-
Define risk in terms of the cash flows from a capital budgeting project. How can determination of the breakeven cash inflow be used to gauge project risk?
-
After a successful first year, Cam and Anna decide to expand Front Row Entertainments operations by becoming a venue operator as well as a tour promoter. A venue operator contracts with promoters to...
-
An election day sample. You want to choose an SRS of 20 of a citys 480 voting precincts for special voting-fraud surveillance on election day. (a) Explain clearly how you would label the 480...
-
1. East Coast Yachts uses a small percentage of preferred stock as a source of financing. In calculating the ratios for the company, should preferred stock be included as part of the companys total...
-
Roberts Company reported net income of $1,268 million in 2021. The weighted average number of common shares outstanding during 2021 was 554 million shares. Roberts paid $40 million in dividends on...
-
Can you conclude whether the numerical value of K for the reaction 2 ICl(g) I 2 (g) + Cl 2 (g) is greater or less than the numerical value of K for the reaction ICl(g) 1/2 I 2 (g) + 1/2 Cl 2 (g)?...
-
(A) Teeth are made principally from the mineral hydroxyapatite, Ca 5 (PO 4 ) 3 OH, which can be dissolved in acidic solution such as that produced by bacteria in the mouth. The reaction that occurs...
-
Express the revenue, R, in terms of p and q. A company is interested in producing and selling a new device called an eyePOD (eyewear personal optical device). The eyePOD is an MP3 and video player...
-
A certain random process \(U(t)\) takes on equally probable values +1 or 0 with changes occurring randomly in time. The probability that \(n\) changes occur in time \(\tau\) is known to be \[...
-
The focal points of the two converging lenses shown in Figure P33.121 are denoted by solid dots for the left lens and open dots for the right lens. Draw a simplified ray diagram to locate the final...
-
Suppose that country A has 9,000 worker hours available for production and that it initially has the technology given by case 3 of Exercise 1. Derive its PPF and determine its exact dimensions. data...
-
The table shows experimental values of the variables x and y. The variables are known to be related by the equation y = a e nx where a and n are constants. a. Draw the graph of ln y against x. b....
-
Construct the Nyquist diagram for a single-degree-of-freedom system with hysteretic damping.
-
Wes Unsel is concerned with control over mail receipts at Wooden Sporting Goods. All mail receipts are opened by Mel Blount. Mel sends the checks to the accounting department, where they are stamped...
-
In July 2013, cnet.com listed the battery life (in hours) and luminous intensity (i. e., screen brightness, in cd/m2) for a sample of tablet computers. We want to know if screen brightness is...
-
Two projects have an identical net present value of $9,000. Are both projects equal in desirability?
-
What are the major disadvantages of the use of the net present value method of analyzing capital investment proposals?
-
What are the major disadvantages of the use of the internal rate of return method of analyzing capital investment proposals?
-
Summarize in your own words Sharps, Treynors, and Jensens Measures for assessing portfolio performance with respect to risk. Assess the portfolio performance of mutual fund VDIGX taking into...
-
Question 1 Slat and Company have recently set up a business which will manufacture and sell a furniture component, the F12 On the 19 August 2021, the company issued 85,000 of share capital for cash....
-
The following is Addison Corporations contribution format income statements for last month. The company has no beginning or ending inventories. A total of 10,000 units were produced and sold last...

Study smarter with the SolutionInn App


