Write equilibrium constant expressions, K p , for the reactions (a) CS(g) + 4H(g) (b) Ag2O(s)
Question:
Write equilibrium constant expressions, Kp, for the reactions
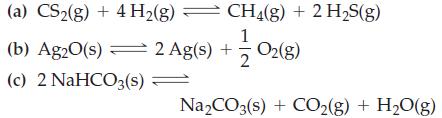
Transcribed Image Text:
(a) CS₂(g) + 4H₂(g) — (b) Ag2O(s) 2 Ag(s) + (c) 2 NaHCO3(s) = CH4(g) + 2 H₂S(g) 12/02 (8) = Na₂CO3(s) + CO₂(g) + H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
The equilibrium constant expressions Kp for the reactions in the image are as follows Reaction 1 CS2...View the full answer

Answered By

YOGENDRA NAILWAL
As I'm a Ph.D. student, so I'm more focussed on my chemistry laboratory. I have qualified two national level exams viz, GATE, and NET JRF (Rank 68). So I'm highly qualified in chemistry subject. Also, I have two years of teaching experience in this subject, which includes college teacher as well as a personal tutor. I can assure you if you hire me on this particular subject, you are never going to regret it.
Best Regards.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Consider the reactions and their respective equilibrium constants: Use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction: NO(g) + Br(g) ...
-
The equilibrium constant for the ethane dehydrogenation reaction, is defined as where P(atm) is the total pressure and yi is the mole fraction of the ith substance in an equilibrium mixture. The...
-
The solution containing no added KNO3 for Figure 7-1 contains 5.0 mM Fe(NO3)3, 5.0 M NaSCN, and 15 mM HNO3. We will use Davies activity coefficients to find the concentrations of all species in the...
-
The condensed financial statements of Soule SpA for the years 2016 and 2017 are presented as follows. SOULE SpA Statements of Financial Position December 31 SOULE SpA Income Statements For the Years...
-
How are risk classes often used to apply RADRs?
-
Advanced Technological Devices Inc. acquired a patent for $120,000. It spent an additional $24,744 defending the patent in legal proceedings. Required: Determine the cost of the patent.
-
Design your own bad sample. Your college wants to gather student opinion about parking for students on campus. It isnt practical to contact all students. (a) Give an example of a way to choose a...
-
Scanlin, Inc., is considering a project that will result in initial after tax cash savings of $1.8 million at the end of the first year, and these savings will grow at a rate of 2 percent per year...
-
please answer these accountingb questions QUESTION 17 Dancer Equipment Company has several divisions that are investment centers. Data for the Sleigh Division and the Trailer Division are shown hero;...
-
For the gas-phase reaction below, the value of K c is 3.4 at 1000 K. What is the value of K p at this temperature? 2 SO2(g) + O2(g) 2 SO3(g)
-
You want to calculate K for the reaction and you have available a K value for the reaction What additional K value do you need, assuming that all K values are at the same temperature? CH4(g) + 2...
-
Discuss major components you would incorporate in a privacy breach response plan. What do you think are the top three components of this type of plan? Why?
-
Recall from Case 1.2 that Auto Concepts is a new division of a large automobile manufacturer that has been slowly losing market share to its competitors. Auto Concepts was created to reclaim the...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located inside the focal length of a diverging lens. \((b)\) Is the image real or virtual? (c) Is it upright or...
-
Show that the ray exiting the block in Figure P33.53 is parallel to the ray entering the block. Data from Figure P33.53
-
The element is subjected to the state of stress shown. If the material is machine steel having a yield stress of \(\sigma_{Y}=750 \mathrm{MPa}\), determine the factor of safety with respect to...
-
Determine the vertical displacement of the ring at point \(B\). \(E I\) is constant. B P A
-
A new accountant at Wyne Inc. is trying to identify which of the amounts shown below should be reported as the current asset "Cash and cash equivalents" in the year-end balance sheet, as of April 30,...
-
The packaging division of a company having considered several alternative package designs for the company's new product has finally brought down their choices to two designs of which only one has to...
-
Battonkill Company, operating at full capacity, sold 112,800 units at a price of $150 per unit during 2010. Its income statement for 2010 is as follows: The division of costs between fixed and...
-
For the coming year, Tolstoy Company anticipates a unit selling price of $100, a unit variable cost of $30, and fixed costs of $2,100,000. Instructions 1. Compute the anticipated break-even sales...
-
Last year, Douthett Inc. had sales of $2,400,000, based on a unit selling price of $600. The variable cost per unit was $440, and fixed costs were $544,000. The maximum sales within Douthetts...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


