Write the balanced chemical equations for reactions that have the following as their standard enthalpy changes. (a)
Question:
Write the balanced chemical equations for reactions that have the following as their standard enthalpy changes.
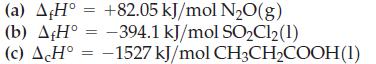
Transcribed Image Text:
(a) AH° +82.05 kJ/mol N₂O(g) (b) AfH° = -394.1 kJ/mol SO₂Cl₂(1) (c) A H° -1527 kJ/mol = CH3CH₂COOH(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Equation a N2Og 32 O2g 2 NO2g Standard enthalpy change H 8205 kJ...View the full answer

Answered By

Robert Mwendwa Nzinga
I am a professional accountant with diverse skills in different fields. I am a great academic writer and article writer. I also possess skills in website development and app development. I have over the years amassed skills in project writing, business planning, human resource administration and tutoring in all business related courses.
4.90+
187+ Reviews
378+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Write the balanced chemical equations for (a) The complete combustion of acetic acid (CH3COOH), the main active ingredient in vinegar (b) The decomposition of solid calcium hydroxide into solid...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
Comparing the chemistry of carbon and silicon. (a) Write balanced chemical equations for the reactions of H 2 O() with CH 4 (forming CO 2 and H 2 ) and SiH 4 (forming SiO 2 and H 2 ). (b) Using...
-
2 3 -4 6,-12,-18 If A = (a) 0 3a 2b 24 (b) -6,4,9 and KA= then the value of k, a,b are respectively (c) -6,-4,-9. (d) -6,12,18
-
The computer workstation furniture manufacturing that Adriana Lopez started in January is progressing well. As of the end of June, Success Systems job cost sheets show the following total costs...
-
The Green Valley Assembly Company assembles consumer electronics products for manufacturers that need temporary extra production capacity. As such, it has periodic product changes. Because the...
-
NEW PROJECT ANALYSIS You must evaluate the purchase of a proposed spectrometer for the R&D department. The base price is $140,000, and it would cost another $30,000 to modify the equipment for...
-
Gary's Parts Shop recorded the following purchases and sales during the past year: Assume that Gary's Parts Shop sold all of the June 15 purchase and 100 cases each from the January 1 beginning...
-
This Question: 10 pts 27 of 28 (0 complete) C-Wireless Wireless needed additional capital to expand, so the business incorporated. The charter from the state of Georgia authorizes C- Wireless to...
-
Illustrate the throughput time and idle time at the two work centers in Solved Problem 15.4 by constructing a time-phased chart. Data From Problem 15.4 Use Johnsons rule to find the optimum sequence...
-
A 1.22 kg piece of iron at 126.5 C is dropped into 981 g water at 22.1 C. The temperature rises to 34.4 C. What will be the final temperature if this same piece of iron at 99.8 C is dropped into 325...
-
Which two of the following statements are false? (a) q V = q P for the reaction N 2 (g) + O 2 (g) 2 NO(g); (b) r H > 0 for an endothermic reaction; (c) By convention, the most stable form of an...
-
Find a basis for the space spanned by the given vectors, v 1 ,....,v 5 . 8 9 -3 -6 0 5 1 -4 4 -4 -9 6 -7 6 8 4 -7 10 4 11 -8 -7
-
BSC-It is important for healthcare leaders to link their departmental balanced scorecard (BSC) to a corporate BSC because it facilitates alignment with the overall strategic objectives of the...
-
Hebert Company adds material at the beginning of production. The following production information is available for March: Beginning Work in Process Inventory (40% complete as to conversion) Started...
-
What modifications would you suggest the leaders of the steel organization when dealing with the use of more efficient technology, carbon emissions, and negative economic impacts in order tomake in...
-
Claxton, Inc. paid a dividend of $ 0 . 9 5 per common share every December from 2 0 0 9 through 2 0 2 3 . The dividend is expected to continue at that level in 2 0 2 4 and 2 0 2 5 . In 2 0 2 6 and...
-
1. Who are two (2) specific examples of effective leaders (who you know personally) who have impacted your life? 2. What made them "effective" leaders? What many specific traits did these leaders...
-
What precautionary procedures should be used when drilling a deep, vertical hole in mild steel when using an ordinary twist drill?
-
Suppose the index goes to 18 percent in year 5. What is the effective cost of the unrestricted ARM?
-
Heartland Companys budgeted sales and budgeted cost of goods sold for the coming year are $144,000,000 and $99,000,000 respectively. Short-term interest rates are expected to average 10%. If...
-
Post-Balance-Sheet Events Keystone Corporation issued its financial statements for the year ended December 31, 2010, on March 10, 2011. The following events took place early in 2011. (a) On January...
-
Post-Balance-Sheet Events or each of the following subsequent (post-balance-sheet) events, indicate whether a company should (a) Adjust the financial statements, (b) Disclose in notes to the...
-
A health insurance policy pays 70% of physical therapy costs after a deductible of $300. In contrast, an HMO charges $20 per visit for physical therapy. How much would a person save with the HMO of...
-
The income statement for the current year is as follows: Sales Cost of goods sold Gross profit Operating expenses: Depreciation expense: Other operating expense Total operating expense Operating...
-
.. yy This is a very simple and inexpensive method. However, it is not precise. Its quality heavily depends on the experience and ability of the buyer to judge the situation. As compared to other...

Study smarter with the SolutionInn App


