You want to test the following proposed mechanism for the oxidation of HBr. You find that the
Question:
You want to test the following proposed mechanism for the oxidation of HBr.
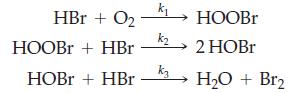
You find that the rate is first order with respect to HBr and to O2. You cannot detect HOBr among the products.
(a) If the proposed mechanism is correct, which must be the rate-determining step?
(b) Can you prove the mechanism from these observations?
(c) Can you disprove the mechanism from these observations?
Transcribed Image Text:
HBr + O₂ HOOBr+ HBr HOBr + HBr k₁ k₂ k3 HOOBr 2 HOBr H₂O + Br₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a If the proposed mechanism is correct which must be the ratedetermining step The ratedetermining st...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
You have studied the gas-phase oxidation of HBr by O2: 4 HBr(g) + O2(g) 2 H2O(g) + 2 Br2(g) You find the reaction to be first order with respect to HBr and first order with respect to O2. You...
-
A department store conducted an experiment to investigate the effects of advertising expenditures on the weekly sales for its men's wear, children's wear, and women's wear departments. Five weeks for...
-
For each of the following categorical statements, identify: (a) the letter label (A, E, I, O) for the statement, (b) the quantity, (c) the quality, (d) the subject and predicate terms, and (e) which...
-
A random variable follows the continuous uniform distribution between 60 and 95. a. Calculate the following probabilities for the distribution: 1. P(x > 63) 2. P(x > 70) 3. P(x > 88) 4. P(x = 75) b....
-
What were the original reasons for the development of the ACRS income tax depreciation method?
-
A 1.00-L aqueous solution contained 6.78 g of barium hydroxide, Ba(OH)2. What was the pH of the solution at 25C?
-
A company growing at an annual rate of 35 percent will double in size in just two years. A company growing at an 18 percent rate will double in size in four years. A company growing by 12 percent...
-
A liquid mixture of 27 wt% acetone and 73 wt% water is to be separated at 25 o C into a raffinate and extract by multistage, steady-state, countercurrent liquid-liquid extraction with a solvent of...
-
I cannot figure out why it wont balance sp e Vinyl Revival Bank Reconciliation Statement June 30, 2020 102964 Balance per bank: Add: deposit in transit deposit in transit 2136 9469 11605 114569...
-
The decomposition of nitric oxide occurs through two parallel reactions: (a) What is the reaction order for these reactions? (b) Which reaction is the slow reaction? (c) If the initial concentration...
-
By taking the reciprocal of both sides of equation 20.36, obtain an expression for 1/V. Using the resulting equation, suggest a strategy for determining the MichaelisMenten constant, K M , and the...
-
List the types of sales data you would expect to find in a sales force automation (SFA) system or customer relationship management (CRM) system. Why is it useful?
-
?In civil engineering, what is the main use of a slump test in concrete technology?
-
Briefly explain what spatial autocorrelation means and what method can be used to measure it
-
Discuss the theoretical implications of adopting biodegradable materials in civil engineering for reducing environmental impact and enhancing sustainability.
-
Analyze the role of civil engineering in coastal erosion management. What are the engineering strategies for shoreline protection, beach nourishment, and coastal infrastructure design to mitigate...
-
Analyze the impact of climate change on civil engineering practices, particularly in the areas of coastal and floodplain management, and discuss strategies for mitigating these impacts
-
Calculate the force required in direct extrusion of 1100-O aluminum from a diameter of 6 in. to 2 in. Assume that the redundant work is 30% of the ideal work of deformation, and the friction work is...
-
The Cholesterol Level data sets give cholesterol levels of heart attack patients. Cholesterol measures are taken 2, 4, and 14 days aft er a patient has suffered a heart attack. Is there a significant...
-
Vladimir Klitschko is preparing a worksheet. Explain to Vladimir how he should extend the following adjusted trial balance accounts to the financial statement columns of the worksheet. Service...
-
The worksheet for Adams Company shows the following in the financial statement columns. J.Q.Adams, Drawing.... $22,000 J.Q.Adams, Capital.... 70,000 Net income....... 29,000 Prepare the closing...
-
Javier Vasquez recently received the following information related to Vasquez Company??s December 31, 2010, balance sheet. Prepare the assets section of Vasquez Company??s classified balancesheet....
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


