A few hydrogen and oxygen molecules are introduced into a container in the quantities depicted in the
Question:
A few hydrogen and oxygen molecules are introduced into a container in the quantities depicted in the following drawing. The gases are then ignited by a spark, causing them to react and form H2O.
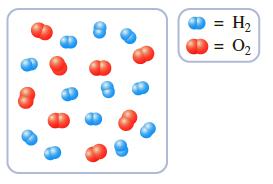
a. What is the maximum number of water molecules that can be formed in the chemical reaction?
b. Draw a molecular level representation of the container’s contents after the chemical reaction.
Transcribed Image Text:
= H2 = O2 %3D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
a Since the balanced chemical equation for the reaction is 2H 2 O 2 2H 2 O in orde...View the full answer

Answered By

Hassan Ali
I am an electrical engineer with Master in Management (Engineering). I have been teaching for more than 10years and still helping a a lot of students online and in person. In addition to that, I not only have theoretical experience but also have practical experience by working on different managerial positions in different companies. Now I am running my own company successfully which I launched in 2019. I can provide complete guidance in the following fields. System engineering management, research and lab reports, power transmission, utilisation and distribution, generators and motors, organizational behaviour, essay writing, general management, digital system design, control system, business and leadership.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the structures of the dipeptides that can be formed from the reaction between the amino acids glycine and lysine.
-
A quantitative definition of solubility is the maximum number of grams of a solute that will dissolve in a given volume of water at a particular temperature. Describe an experiment that would enable...
-
What is the maximum number of PRIMARY KEY constraints allowed for a table? a. 1 b. 2 c. 30 d. 255
-
True Or False Death benefits are used to compensate the deceaseds family for pain and suffering.
-
Sunnydale Farm includes 240 acres of cropland. The farm owner wishes to plant this acreage in corn and oats. The profit per acre in corn production is $40 and in oats is $30. A total of 320 hr of...
-
What is the contingency view of knowledge management? How does it differ from the universalistic view of knowledge management?
-
Quatro Corp. engages solely in manufacturing operations. The following data pertain to the operat ing segments for the current year: Operating LO3 Total Segment Revenues Profit Assets at 12/31 A. . ....
-
A business provides its employees with varying amounts of vacation per year, depending on the length of employment. The estimated amount of the current year's vacation pay is $42,000. a. Journalize...
-
The changes in each balance sheet account for Carver Corporation during the year just completed are as follows: Increase Decrease Cash and cash equivalents $ 3,090 Accounts receivable $ 5,500...
-
Required: 1 (20 pts) Conduct a 2-year weighted annual sales forecast for the years 2023-2027. Place your results on the Sales line of the Milestone 1 Sales Forecast tab in the highlighted area. 2 (20...
-
Find the formula weights of the following substances to three significant figures. a. Formaldehyde, CH 2 O b. Sulfur dioxide, SO 2 c. Sodium carbonate, Na 2 CO 3 d. Lead(II) phosphate, Pb 3 (PO 4 ) 2
-
You perform the hypothetical reaction of an element, X 2 (g), with another element, Y(g), to produce XY(g). a. Write the balanced chemical equation for the reaction. b. If X 2 and Y were mixed in the...
-
a. Are there any going concern issues for HCHG? Explain. If so, what are the mitigating circumstances? b. How will you recommend that the issues be handled in the financial statements and the audit...
-
X Calculate the reaction rate at various conversions, as shown below: FAO -TA -TA 0.2 0.8 Considering that for a PFR: dx V = FAO What is the conversion reached after the 50 m of this PFR?
-
For decades, leaders have talked about flexible working options, yet only few companies were consistently using these practices prior to the global health crisis of 2020. In March 2020, organizations...
-
Manatee Corp. has developed standard costs based on a predicted operating level of 352,000 units of production, which is 80% of capacity. Variable overhead is $281,600 at this level of activity, or...
-
When leaders are facing a crisis or an opportunity, generally, they tend to fall back on the leadership style that has worked for them in the past. Discuss with examples the options that would help...
-
Data Table the pasteet dollar -X Total sales revenues 2 Number of units produced and sold 500,000 units Selling price nt. $ 230,000 te Operating Income Total Investment in assets Variable cost per...
-
The following program is legal under Javas syntax rules, but it is difficult to read because of its layout and lack of comments. Reformat it using the rules given in this chapter, and add a comment...
-
Suppose that the electrical potential at the point (x, y, z) is E(x, y, z) = x + y - 2z. What is the direction of the acceleration at the point (1,3,2)?
-
Compounds of boron with hydrogen are called boranes. One of these boranes has the empirical formula BH3 and a molecular mass of 28 amu. What is its molecular formula?
-
Oxalic acid is a toxic substance used by laundries to remove rust stains. Its composition is 26.7% C, 2.2% H, and 71.1% O (by mass), and its molecular mass is 90 amu. What is its molecular formula?
-
Adipic acid is used in the manufacture of nylon. The composition of the acid is 49.3% C, 6.9% H, and 43.8% O (by mass), and the molecular mass is 146 amu. What is the molecular formula?...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


