C 6 H 4 NH 2 COOH, para-aminobenzoic acid (PABA), is used in some sunscreen agents. Calculate
Question:
C6H4NH2COOH, para-aminobenzoic acid (PABA), is used in some sunscreen agents. Calculate the concentrations of hydronium ion and para-aminobenzoate ion, C6H4NH2COO-, in a 0.080 M solution of the acid. The value of K a is 2.2 × 10-5.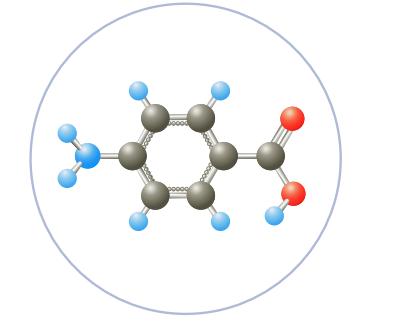
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
To solve assemble a table of starting change and equilibrium concen...View the full answer

Answered By

Ajeet Singh
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students. Areas of interest: Business, accounting, Project management, sociology, technology, computers, English, linguistics, media, philosophy, political science, statistics, data science, Excel, psychology, art, history, health education, gender studies, cultural studies, ethics, religion. I am also decent with math(s) & Programming. If you have a project you think I can take on, please feel welcome to invite me, and I'm going to check it out!
5.00+
4+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
C6H4NH2COOH, para-aminobenzoic acid (PABA), is used in some sunscreen agents. Calculate the concentrations of hydronium ion and para-aminobenzoate ion, C6H4NH2COO, in a 0.055 M solution of the acid....
-
A solution of barium hydroxide at 25C is 0.125 M Ba(OH)2. What are the concentrations of hydronium ion and hydroxide ion in the solution?
-
Barbituric acid, HC4H3N2O3, is used to prepare various barbiturate drugs (used as sedatives). Calculate the concentrations of hydronium ion and barbiturate ion in a 0.10 M solution of the acid. The...
-
Show that if powers of x greater than x 5 are neglected. In sin x X =-=-x-x 180
-
Refer to the International Conference on Web Intelligence and Intelligent Agent Technology (2010) study of how social media (e.g., Twitter.com) may influence the products consumers buy, Recall that...
-
Examine the development and use of selection criteria? LO1
-
Reacting to risks. National newspapers such as USA Today and the New York Times carry many more stories about deaths from airplane crashes than about deaths from automobile crashes. Auto accidents...
-
Matyas Company completed the salary and wage payroll for April 2014. The payroll provided the following details: Required: 1. Prepare the journal entry to record the payroll for April, including...
-
AaBBC AaBbcc AaBbc AaBbccc Add 1 Normal 1 No Spac... Heading 1 Heading 2 Title Replace Select Dictate Sensitivity Paragraph Styles Editing Voice Sensitivity Principles of Accounting I BUSI 201-01...
-
Following is a labeled flowchart for a steady-state two-unit process with boundaries shown to denote subsystems about which balances can be taken. State the maximum number of balances that can be...
-
Formic acid, HCHO 2 , is used to make methyl formate (a fumigant for dried fruit) and ethyl formate (an artificial rum flavor). What is the pH of a 0.12 M solution of formic acid? What is the degree...
-
A solution of acetic acid, HC 2 H 3 O 2 , on a laboratory shelf was of undetermined concentration. If the pH of the solution was found to be 2.57, what was the concentration of the acetic acid? The K...
-
Complete the analysis started in Example 1 by providing the details of the linearization about the point (-1, 0) for x' = y, y' = - y + x - x3.
-
You are the manager of internal audit of Coverit Corporation, a large insurance company. One day you receive an urgent letter from the controller expressing his concerns about some organizational...
-
Daintree Ltd. is a large retailer that operates department stores in all major cities throughout Australia. Recently it has expanded its operations into Southeast Asia. Although each store operates...
-
Draw two points P and Q. Then sketch PQ. Add a point R on the ray so that Q is between P and R. C D A B FL E
-
Hypothesis testing and testing claims with confidence intervals are two different approaches that lead to the same conclusion. In the following activities, you will compare and contrast those two...
-
The following system of periodic tasks is scheduled and executed according to a cyclic schedule. Draw an execution trace (timeline) showing two occurances of each task. Ti ei Pi 1 8 T2 4 15 T3 3 20...
-
A steel rod of 0.5 cm diameter and 10 m length is stretched 3 cm. Youngs modulus for this steel is 21 kN/cm 2 . How much work, in kJ, is required to stretch this rod?
-
Given the table below, about how much force does the rocket engine exert on the 4.0 kg payload? Distance traveled with rocket engine firing (m) Payload final velocity (m/s) 500 320 490 310 1020 450...
-
The following data were collected for the reaction A(g) + B(g) Products. a. Determine the rate law for this reaction. b. Calculate the rate constant. c. Calculate the rate when [A] = 0.200 M and [B]...
-
Dinitrogen pentoxide, N2O5, undergoes first-order decomposition in chloroform solvent to yield NO2 and O2. The rate constant at 45oC is 6.2 104/min. Calculate the volume of O2 obtained from the...
-
Hydrogen peroxide undergoes a first-order decomposition to water and O2 in aqueous solution. The rate constant at 25oC is 7.40 104/s. Calculate the volume of O2 obtained from the decomposition...
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


