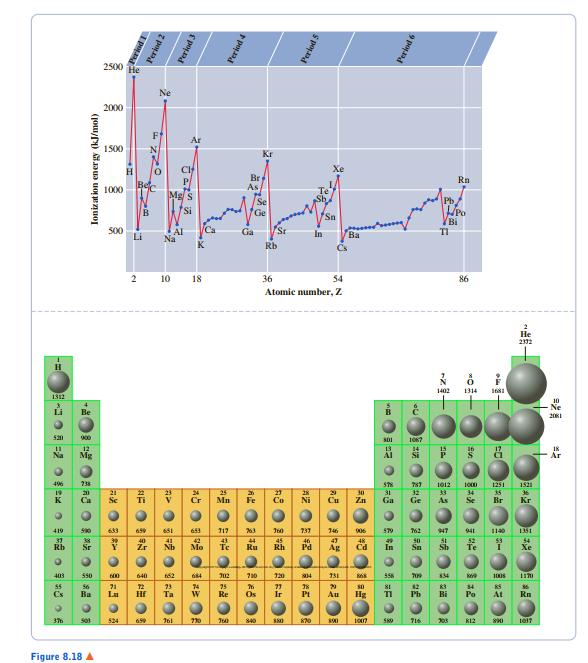
From Figure 8.18, predict the first ionization energy of francium (Z = 87). 2500 He 2000 *
Question:
From Figure 8.18, predict the first ionization energy of francium (Z = 87).
Transcribed Image Text:
2500 He 2000 * 1500 Kr Br As Rn E 1000 Bi S00 Li Ca Sr Na In Ba K Rb Cs 10 18 36 54 86 Atomic number, Z -Ne 吕 550 1008 At 524 730 J0 s40 S0 203 K12 Figure 8.18 A pona Period 2 Period 3 Ionizat ion energy (kJ/mol) Period 4 Perlod 5 つ=2の o 6 Perlod 6 AZ 中 一
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
The ionization ...View the full answer

Answered By

Wonder Dzidzormenu
As a professional accountant and a teacher, I explain account ing concepts in a more practical way that makes students more connected to the subject.
With over 10 years of teaching accounting , I offer a well constructed , easily understood and in-depth explanations to students questions.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
From Figure 8.18, predict the first ionization energy of ununseptium (Z = 117). 2500 He 2000 * 1500 Kr Br As Rn E 1000 Bi S00 Li Ca Sr Na In Ba K Rb Cs 10 18 36 54 86 Atomic number, Z -Ne 550 1008...
-
The first ionization energy of the chlorine atom is 1251 kJ/mol. Without looking at Figure 8.18, state which of the following values would be the more likely ionization energy for the iodine atom....
-
The first ionization energy of the oxygen molecule is the energy required for the following process: O2 (g) O2+ (g) + e- The energy needed for this process is 1175kJ/mol, very similar to the first...
-
A sample consisting of 1.00mol Ar is expanded isothermally at 20 C from 10.0dm 3 to 30.0dm 3 (i) Reversibly, (ii) Against a constant external pressure equal to the final pressure of the gas, (iii)...
-
Sport utility vehicles (SUVs), vans, and pickups are generally considered to be more prone to roll over than cars. In 1997, 24.0% of all highway fatalities involved rollovers; 15.8% of all fatalities...
-
(Imputation of Interest) Presented below are two independent situations: (a) On January 1, 2008, Robin Wright Inc. purchased land that had an assessed value of $350,000 at the time of purchase. A...
-
M/s Anil & Company, a firm of building contractors undertook a contract for construction of a commercial complex on 1st January 1997. The following was the expenditure on the contract for Rs...
-
The Bettinghaus Corporation began business on January 2, 2010, with five employees. It created a sick leave and vacation policy stated as follows: Each employee is allowed eight days of paid sick...
-
Successful companies generate the greatest percentage of cash from: borrowing money sale of fixed assets O sale of goods and services O sale of investments The sales of PB&J Window Treatments for...
-
There are 2 shinobis with chakra levels 5 and 10 respectively and the desired sum of chakra levels is utmost 15 Starting with ke0, suy of chakra levels after attack max(5-0,0) + max(10- 0,0) 5+10 15....
-
What property of an atom does nuclear magnetic resonance depend on? What frequency range of the electromagnetic spectrum does NMR use?
-
For eka-lead, predict the electron configuration, whether the element is a metal or nonmetal, and the formula of an oxide.
-
Hasbro and Mattel, Inc., are the two largest toy companies in North America. Condensed liabilities and stockholders' equity from a recent balance sheet are shown for each company as follows (in...
-
Write down at leastfive items (durable goods, not food) that you purchase and their sourcing (where each is from). For example, a shirt may be assembled in China, designed in the US, and made from...
-
I agree that overtime can be tricky in different countries. As we've seen with piecework, it is hard to implement it in countries where people are not motivated to work past their regular hours, even...
-
time (in seconds). Find a formula for 1, if V = 5t(t Suppose that an object's acceleration function is given by a = 4t+ 6. The object's initial velocity is 4, and the initi position is 9. Find the...
-
Determine the specific major and foundational managerial discoveries and findings from each era as most pivotal for management evolution (Early Management Era, Social Management Era, Scientific...
-
Identify the three major pricing strategies and discuss the important key factors that impact setting prices. Explain what time of pricing strategy your assigned brand uses and why you believe this...
-
Predict the geometries of the following ions, using the VSEPR model. a. GeF 5 b. AsF 6 c. BrF 2 d. BrF 4
-
A firm has the following balance sheet: Assets Cash Accounts receivable Inventory Plant and equipment $ 15,000 150,000 92,000 170,000 $427,000 Liabilities and Equity Accounts payable Long-term debt...
-
In this problem you need to draw two pictures of solutions in beakers at different points in time. Time zero (t = 0) will be the hypothetical instant at which the reactants dissolve in the solution...
-
You come across a beaker that contains water, aqueous ammonium acetate, and a precipitate of calcium phosphate.
-
Equal quantities of the hypothetical strong acid HX, weak acid HA, and weak base BZ, are added to separate beakers of water, producing the solutions depicted in the drawings. In the drawings, the...
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


