Indicate the molecular geometry around each carbon atom in the compound H;CCH=CHCH,CCH,COOH
Question:
Indicate the molecular geometry around each carbon atom in the compound
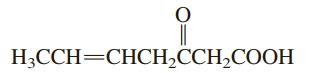
Transcribed Image Text:
H;CCH=CHCH,CCH,COOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
Numbering the seven carbon atoms from left to right the ...View the full answer

Answered By

HARSH RANJAN
Taken classes at college to graduates, Also worked as an expert to a freelancer online question-solving portal for more than 8 months with an average rating greater than 4.2 out of 5.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following samples has a larger mass? O 19.3 g of Au 8.92 10 atoms of Pt Identify the molecular geometry around the three selected atoms. Treat that atoms as the central atom for each...
-
Search Textbo a. chloroform, CHCI3 (carbon is central atom) Lewis structure Total number of electron groups around the central atom Electron geometry Molecular shape b. Carbon tetrachloride, CCI4...
-
The lactic acid molecule, CH3CH (OH) COOH, gives sour milk its unpleasant, sour taste. (a) Draw the Lewis structure for the molecule, assuming that carbon always forms four bonds in its stable...
-
What is the coefficient of sliding friction and what is a representative value for this coefficient for the brittle crust?
-
A coagulation-microfiltration process for removing bacteria from water was investigated in Environmental Science & Engineering (Sept. 1, 2000). Chemical engineers at Seoul National University...
-
21 A listed company in which you have invested a lot of your personal wealth has just filed for insolvency. Using Altmans Z-score analysis, would you have been able to predict its financial distress?...
-
Explain the relationship between price and quantity sold.
-
On January 1, 2007 the Stimpson Company sells land to Barker Company for $2.5 million, then immediately leases it back. The relevant information is as follows: 1. The land was carried on Stimpsons...
-
A firm has a tax burden ratio of 0.95, a leverage ratio of 1.6, an interest burden of 0.6, and a return on sales of 9%. The firm generates $2.5 in sales per dollar of assets. What is the firm's ROE?...
-
Should the handshaking in SSL occur before or after the three-way handshaking in TCP? Can they be combined? Explain.
-
Draw the cis and trans isomers of the compound 3-methyl-3-hexene.
-
Complete and balance the reaction for each of the following undergoing a combustion reaction. a. 2-methylpentane b. 2,2-dimethylpropane c. 3-methyl-3-hexene d. 3-ethyl-3-propanol e. ethylbenzene
-
Is Figure 10.1 a realistic model of the evaluation and control process?
-
who do you think sets the underlying ethical standards when the law is fuzzy on an issue? as business and societal issues develop in the future, how does your opinion in this area inform your...
-
how do i introduce low risk high reward for a new medical assistant supervisor role in an organization?
-
How do individual differences in cognitive styles, such as analytical versus intuitive thinking, impact problem-solving approaches and decision-making processes within teams ?
-
In Russian government, do you think that Russian Military Performance is good in warfare against Ukraine? Explain.
-
Why do you think the competing values framework is important to an organization's effectiveness? Describe the four profiles of the competing values framework. Identify one of the profiles and provide...
-
In problem use the improved Eulers method to obtain a four-decimal approximation of the indicated value. First use h = 0.1 and then use h = 0.05. y' = x + y 2 , y(0) = 0; y(0.5)
-
How can a promoter avoid personal liability for pre-incorporation contracts?
-
a. When 0.10 mol of the ionic solid NaX, where X is an unknown anion, is dissolved in enough water to make 1.0 L of Concept Explorations solution, the pH of the solution is 9.12. When 0.10 mol of the...
-
Which of the following beakers best represents a container of a weak acid, HA, in water? (Water molecules have been omitted for clarity.) ,- = A- = HA A
-
You have 0.10-mol samples of three acids identified simply as HX, HY, and HZ. For each acid, you make up 0.10 M solutions by adding sufficient water to each of the acid samples. When you measure the...
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


