Methanol, CH 3 OH, is prepared industrially from the gas-phase catalytic balanced reaction that has been depicted
Question:
Methanol, CH3OH, is prepared industrially from the gas-phase catalytic balanced reaction that has been depicted here using molecular models.
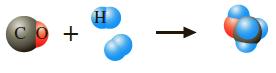
In a laboratory test, a reaction vessel was filled with 41.1 g CO and 10.2 g H2. How many grams of methanol would be produced in a complete reaction? Which reactant remains unconsumed at the end of the reaction? How many grams of it remain?
H Co+
Step by Step Answer:
First determine whether CO or H 2 is the limiting reactant by calculatin...View the full answer



Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
Methanol, CH3OH, is prepared industrially from the gasphase catalytic balanced reaction that has been depicted here using molecular models. In a laboratory test, a reaction vessel was filled with...
-
Methanol is prepared industrially from synthesis gas (CO and H 2 ). CO(g) + 2H 2 (g) CH 3 OH(g); H = 21.7 kcal Would the fraction of methanol obtained at equilibrium be increased by raising the...
-
Methanol is prepared industrially from synthesis gas (CO and H 2 ). CO(g) + 2H 2 (g) CH 3 OH(g); H = 21.7 kcal Would the fraction of methanol obtained at equilibrium be increased by raising the...
-
When a cosmetic manufacturer tests the market to determine how many women will buy eyeliner that has been tested for safety without subjecting animals to injury, is it involved in a descriptive...
-
Kayla sold a total of 145 Italian sausages and hot dogs from her curbside pushcart and collected $242.05. She sold 45 more hot dogs than sausages. How many of each did she sell? CENTR
-
What are absorptive capacity and shared domain knowledge? How do they relate to common knowledge?
-
Many companies make quarterly reports available on their corporate Internet home page. Quarterly reports also can be accessed through the SECs EDGAR system at www.sec.gov (under Forms, search for...
-
Inez has a specific set of plans to build a sailboat. The plans are detailed, and any boatbuilder can construct the boat. Inez secures bids, and the low bid is made by the Whale of a Boat Corp. Inez...
-
CAPM 4. The fair expected return of the stock is 15%. Find the market risk premium if the riskfree Rate is 6% and the stocks beta is 0.9. What is the market expected return?
-
Perform particle motion simulation in framework of particle-in-cell approach. Calculate the trajectory of an electron using the parameters given in Table.1. All other components are zero. X...
-
Nickel(II) chloride reacts with sodium phosphate to precipitate nickel(II) phosphate. 3NiCl 2 (aq) + 2Na 3 PO 4 (aq) Ni 3 (PO 4 ) 2 (s) + 6NaCl(aq) How many moles of nickel(II) chloride are needed...
-
Calcium carbide, CaC 2 , used to produce acetylene, C 2 H 2 , is prepared by heating calcium oxide, CaO, and carbon, C, to high temperature. CaO(s) + 3C(s) CaC 2 (s) + CO(g) If a mixture contains...
-
GOME (www.GOME.com.hk), Best Buy, and RadioShack are competitors in the global marketplace. Following are selected data from each company. Required 1. Compute GOMEs return on total assets, and its...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 C. Determine the magnitude and direction of the electric field along the axis of the rod at a point 32.0 cm from its center....
-
Hello need help with this problem. The transactions relating to the formation of Blue Company Stores Incorporated, and its first month of operations follow. a. The firm was organized and the...
-
At the beginning of the year, the net assets of Shannon Company were $492,600. The only transactions affecting stockholders equity during the year were net income of $70,200 and dividends of $15,400....
-
The claim is that smokers have a mean cotinine level greater than the level of 2.84 ng/mL found for nonsmokers. (Cotinine is used as a biomarker for exposure to nicotine.) The sample size is n = 739...
-
Suppose you have an int variable called number . What Java expression produces the last digit of the number (the 1s place)?
-
A 20-cm-square vertical plate is heated to a temperature of 30oC and submerged in glycerin at 10oC. Calculate the heat lost from both sides of the plate.
-
Classify each of the following reactions as a combination reaction, decomposition reaction, displacement reaction, or combustion reaction. a. When solid calcium oxide, CaO, is exposed to gaseous...
-
Consider the reaction of all pairs of the following compounds in water solution: Ba(OH)2, Pb(NO3)2, H2SO4, NaNO3, MgSO4. a. Which pair (or pairs) forms one insoluble compound and one soluble compound...
-
Consider the reaction of all pairs of the following compounds in water solution: Sr(OH)2, AgNO3, H3PO4, KNO3, CuSO4. a. Which pair (or pairs) forms one insoluble compound and one soluble compound...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


