Shown below is a diagram depicting the enthalpy change of a chemical reaction run at constant pressure.
Question:
Shown below is a diagram depicting the enthalpy change of a chemical reaction run at constant pressure.
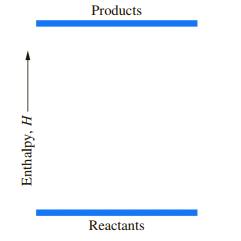
a. Is the reaction exothermic or endothermic?
b. What is the sign of ∆H?
c. What is the sign of q?
d. If the reaction does no work, what is the sign of ∆E for this process?
Transcribed Image Text:
Products Reactants Enthalpy, H-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
a Because the enthalpy increases when going from rea...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Thermodynamics and Thermochemistry 105 [Useful information : 1 J= 1kg m's-2, 1 Pa = 1 kg m s; 1 bar 10 Pa] Given that AS (A +C) = 50 eu AS C + D) = 30 eu AS (D - B) = -20 euwhere, eu is entropy unit...
-
14 Thermodynamics and Thermochemistry . The reaction, MgO(s) + C(s) Mg(s) + CO(g ) 18 The entropy change associated with the conversion of 1 kg of ice at 273 K to water vapours at 383 K is for which...
-
1. Which has maximum internal energy at 298 K? a) helium gas c) ozone gas d) equal b) oxygen gas For a gas having molar mass M, specific heat at constant pressure can be given as: YR a) M(Y-1) YRM a...
-
In Exercises find the derivative of the function by the limit process. f(x) = x - 4x + 5
-
The file Energy contains the per capita energy consumption, in kilowatt-hours, for each of the 50 states and the District of Columbia during a recent year. a. Compute the mean, variance, and standard...
-
1 What went well during that task, which we should try to repeat?
-
12. Suppose S = \($100\), K = \($95\), = 30%, r = 0.08, = 0.03, and T = 0.75. Using the technique in the previous problem, compute the Greek measure corresponding to a change in the dividend yield....
-
Suppose the current price of gold is $1,200 an ounce. Hotshot Consultants advises you that gold prices will increase at an average rate of 12% for the next two years. After that the growth rate will...
-
One amount is missing in the following trial balance of proprietary accounts, and another is missing from the trial balance of budgetary accounts of the Save Our Resources Commission of the federal...
-
Complete Keith's tax return including all required schedules and forms using prince edward island as province, using the fillable forms package. Taxpayers Information Taxpayer #1 Name: Keith Dox...
-
Hydrogen sulfide, H 2 S, is produced during decomposition of organic matter. When 0.5000 mol H 2 S burns to produce SO 2 (g) and H 2 O(l), 281.0 kJ of heat is released. What is this heat in...
-
Consider the following specific heats of metals. Metal .......................Specific Heat copper...................... 0.385 J/(gC) magnesium ...............1.02 J/(gC) mercury...
-
What is the role of the U.S. Federal Reserve System?
-
Case Study : While it might be easy to see the negative effects on the environment from car emissions or the waste we produce, fewer people think about the effects of discarded clothes on the...
-
CompanyWeek 8 Assignment - Financial Statement Analysis Overview In this assignment, you will take your work with financial statements to the next level. You will analyze financial statements similar...
-
In Exercises 9-12, assume that 100 births are randomly selected. Use subjective judgment to describe the given number of girls as (a) significantly low, (b) significantly high, or (c) neither...
-
Which of the following is not included in the cash flow statement? a. Cash from short-term investments b. Cash from operations c. Cash from the balance sheet d. Cash from capital financing Which of...
-
Case Study Chapter 13B Pharm - Saved Case Study Chapter 13 Central Nervous System Stimulants and Related Drugs Nancy has been unsuccessful in preventing migraine headaches and has been prescribed a...
-
A solution was prepared by dissolving 0.800 g of sulfur, S8, in 100.0 g of acetic acid, HC 2 H 3 O 2 . Calculate the freezing point and boiling point of the solution.
-
Rowland Textile Inc. manufactures two products: sweatshirts and T-shirts. The manufacturing process involves two activities: cutting and sewing. Expected overhead costs and cost drivers are as...
-
A vessel containing 39.5 cm3 of helium gas at 25oC and 106 kPa was inverted and placed in cold ethanol. As the gas contracted, ethanol was forced into the vessel to maintain the same pressure of...
-
The volume occupied by a gas depends linearly on degrees Celsius at constant pressure, but it is not directly proportional to degrees Celsius. However, it is directly proportional to kelvins. What is...
-
A sample of 62.3 cm3 of argon gas at 18oC was contained at a pressure of 155 kPa in a J shaped tube with mercury. Later the temperature changed. When the mercury level was adjusted to give the same...
-
Construction of consumer price index number for the given goods and services. Item Weight in % Base period price Current period price Food 35 150 145 Fuel 10 25 23 Cloth 20 75 65 Rent 15 30 30 Misc....
-
Gammaro Corporation has found that 80% of its sales in any given month are credit sales, while the remainder are cash sales of the credit sales, Gammaro Corporation has experienced the following...
-
Swifty Company estimates that 2022 sales will be $43,200 in quarter 1,$51,840 in quarter 2 , and $62,640 in quarter 3 , Cost of goods sold is 50% of sales. Management desires to have ending...

Study smarter with the SolutionInn App


