Sodium azide, NaN 3 , undergoes the reaction NaN 3 (s) 2Na(s) + 3N 2 (g).
Question:
Sodium azide, NaN3, undergoes the reaction NaN3(s) → 2Na(s) + 3N2(g). Because this reaction is very fast and produces nitrogen gas, NaN3 is used to inflate airplane escape chutes. Sodium azide can be produced through two reaction steps.
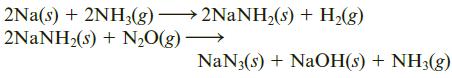
Starting with 1.0 kg of Na, 6.0 kg of NH3, and 1.0 kg of N2O, what is the maximum mass (kg) of sodium azide that can be produced?
Transcribed Image Text:
2Na(s) + 2NH3(g) 2NANH,(s) + H2(g) 2NANH2(s) + N,O(g) - NaN3(s) + NaOH(s) + NH3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
This problem is best approached by first combining the two steps given in the s...View the full answer

Answered By

Jehal Shah
I believe everyone should try to be strong at logic and have good reading habit. Because If you possess these two skills, no matter what difficult situation is, you will definitely find a perfect solution out of it. While logical ability gives you to understand complex problems and concepts quite easily, reading habit gives you an open mind and holistic approach to see much bigger picture.
So guys, I always try to explain any concept keeping these two points in my mind. So that you will never forget any more importantly get bored.
Last but not the least, I am finance enthusiast. Big fan of Warren buffet for long term focus investing approach. On the same side derivatives is the segment I possess expertise.
If you have any finacne related doubt, do reach me out.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In each of the following indicate which reaction will occur faster. Explain your reasoning. (a) CH3CH2CH2CH2Br or CH3CH2CH2CH2I with sodium cyanide in dimethyl sulfoxide (b) 1-Chloro-2-methylbutane...
-
In each of the following indicate which reaction will occur faster. Explain your reasoning. (a) CH3CH2CH2CH2Br or CH3CH2CH2CH2I with sodium cyanide in dimethyl sulfoxide (b) 1-Chloro-2-methylbutane...
-
Starting with sodium azide as your source of nitrogen and using any other reagents of your choice, show how you would prepare each of the compounds in Problem 23.18. Compounds in 23.18 (a) (b) (c)...
-
What is the effect of a viscosity (competence) difference between strain markers and the matrix?
-
A bridge with a semielliptical arch spans a river as shown here. What is the clearance 6 ft from the riverbank? 14 ft 50 ft
-
18.19 What is the chief disadvantage of ROI as an investmentcenter performance measure? How does the residualincome measure eliminate this disadvantage?
-
What is an operating segment? LO3
-
Select the best answer to the following multiple-choice questions: 1. Which of the following adjustments would likely be made when moving from governmental funds financial statements to...
-
The following is the financial statement of Executive Dynamic Mattress for the year ended December 2 0 2 1 . INCOME STATEMENT, 2 0 2 1 Revenue $ 2 , 0 0 0 . 0 Cost of goods sold 1 , 8 0 0 . 0...
-
On December 31, Year 1, Kelly Corporation of Toronto paid 13.7 million Libyan dinars (LD) for 100% of the outstanding common shares of Arkenu Company of Libya. On this date, the fair values of...
-
Dinotrogen monoxide, commonly known as laughing gas, can be obtained by cautiously warming ammonium nitrate according to the equation NH 4 NO 3 (s) N 2 O(g) + 2H 2 O(g) If the reaction has a 75%...
-
A sample containing only boron and fluorine was decomposed yielding 4.75 mg of boron and 17.5 mL of fluorine gas (density = 1.43 g/L). What is the empirical formula of the sample compound?
-
Figure a shows a thin tube in which a finite potential trap has been set up where V2 = 0 V. An electron is shown traveling rightward toward the trap, in a region with a voltage of V1 = ?? 9.00 V,...
-
Discuss the Competitive Markets and Externalities simulations (both with and without policy interventions) . What impact do policy interventions have on the supply and demand equilibrium for a...
-
The best consultant to fix issue number one is Frederick Taylor who is credited with creating the scientific management movement (Lumen, n.d.). Since Taylor's work focused on how a process could be...
-
1. Which Pepsico products are growing faster than soft drinks (why) and by what percentage? 2. Why do the fastest growing products experience a more complex supply chain? Explain. 3. What are some of...
-
Use BLUF (Bottom Line UP Front) or Brief for answering the following questions: 1) There are a number of InfoSec frameworks / models available in industry. A. What is an InfoSec framework / model? B....
-
An introduction to organizational structure. Topics such as alternative organizational structures, the reciprocal relationship between multinational strategy and structure, and how recourses affect...
-
Write a program that produces as output the words of the song, Bought Me a Cat. Use methods for each verse and for repeated text.
-
Show, if u(x, y) and v(x, y) are harmonic functions, that u + v must be a harmonic function but that uv need not be a harmonic function. Is e"e" a harmonic function?
-
Hydrogen peroxide, H2O2, is a colorless liquid. A concentrated solution of it is used as a source of oxygen for rocket propellant fuels. Dilute aqueous solutions are used as a bleach. Analysis of a...
-
Nitric acid, HNO3, is a colorless, corrosive liquid used in the manufacture of nitrogen fertilizers and explosives. In an experiment to develop new explosives for mining operations, a sample...
-
Hydrogen cyanide, HCN, is a volatile, colorless liquid with the odor of certain fruit pits (such as peach and cherry pits). The compound is highly poisonous. How many molecules are there in 56 mg...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...

Study smarter with the SolutionInn App


