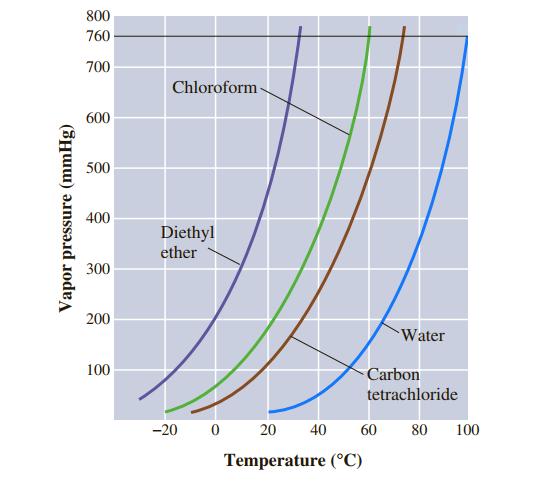
Use Figure 11.7 to estimate the boiling point of carbon tetrachloride, CCl 4, under an external pressure
Question:
Use Figure 11.7 to estimate the boiling point of carbon tetrachloride, CCl4, under an external pressure of 250 mmHg.
Transcribed Image Text:
800 760 700 Chloroform- 600 500 400 Diethyl ether 300 200 Water 100 Carbon tetrachloride -20 20 40 60 80 100 Temperature (°C) Vapor pressure (mmHg)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
Dropping a line from the inter...View the full answer

Answered By

Antony Mutonga
I am a professional educator and writer with exceptional skills in assisting bloggers and other specializations that necessitate a fantastic writer. One of the most significant parts of being the best is that I have provided excellent service to a large number of clients. With my exceptional abilities, I have amassed a large number of references, allowing me to continue working as a respected and admired writer. As a skilled content writer, I am also a reputable IT writer with the necessary talents to turn papers into exceptional results.
4.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use Figure 11.7 to estimate the boiling point of diethyl ether, (C 2 H 5 ) 2 O, under an external pressure of 470 mmHg. 800 760 700 Chloroform- 600 500 400 Diethyl ether 300 200 Water 100 Carbon...
-
Using the vapor-pressure curves in Figure 11.25, (a) Estimate the boiling point of ethanol at an external pressure of 200 torr; (b) Estimate the external pressure at which ethanol will boil at 60 oC;...
-
Use Figure 10.8 to estimate the boiling point of ethanol at 400 torr.
-
a. Find the nth-order Taylor polynomials for the given function centered at the given point a, for n = 0, 1, and 2.b. Graph the Taylor polynomials and the function. f(x) = ln x, a e =
-
Suppose FRM, Inc., issued a zero coupon, equity index-linked note with a five-year maturity. The par value is $1,000, and the coupon payment is stated as 75 percent of the equity index return or as...
-
Extinct New Zealand birds. Refer to the Evolutionary Ecology Research (July 2003) study of the patterns of extinction in the New Zealand bird population, presented in Exercise 2.24 (p. 70). Consider...
-
Length of pregnancies. The length of human pregnancies from conception to birth varies according to a distribution that is approximately Normal with mean 266 days and standard deviation 16 days. Use...
-
Downward demand spiral Spirelli Company is about to enter the highly competitive personal electronics market with a new optical reader. In anticipation of future growth, the company has leased a...
-
Circles Inc. currently pays a dividend of $3 per share (i.e. D0 = $3), and this dividend is expected to grow at a constant rate of 5 percent forever. The company's stock has a required rate of return...
-
a. Draw a UML class diagram that describes the Beach Dudes sales and collection process. b. Using Microsoft Access, implement a relational database from your UML class diagram. Identify at least...
-
Chloroform, CHCl 3 , a volatile liquid, was once used as an anesthetic but has been replaced by safer compounds. Chloroform boils at 61.7C and has a heat of vaporization of 31.4 kJ/mol. What is its...
-
An element crystallizes with a simple cubic lattice with atoms at all the lattice points. If the radius of the atom is 200. pm, what is the volume of the unit cell? a. 8.00 10 6 pm 3 b. 6.40 10 7...
-
Define business intelligence (IB). Describe the relationship between Business Intelligence applications and tools in IT industry. Define IBM Cognos Intelligence, Business activity monitoring (BAM),...
-
Low Desert Pottery works makes a variety of pottery products that it sells to retailers. The company uses a job-order costing system in which departmental predetermined overhead rates are used to...
-
ASSESSMENT CPCCBC5002A Monitor costing systems on medium rise building and construction projects Please provide answer to Part 2 - Monitor expenditure for a medium-rise project as per below...
-
Questions 6-8 refer to the same problem A sinusoidal wave with wavelength 2 m and amplitude 5 mm is traveling along the x axis. The wave is traveling in the -x direction at a speed of 2m/s At t = Os,...
-
Consider a circuit where one or more capacitors is discharged through a light bulb filament with a resistance of 3.0 0.3 . Assume that the resistance of the filament is constant (to within the stated...
-
3. For a vibrating string of length with fixed ends, each mode of vibration can be written as where wk ux(x, t) = M* sin(wxt + k) sin(x) and Mk, Ok are determined by initial conditions. For all k >...
-
In a hypothetical universe, the quantum numbers for an atomic orbital follow these rules: n = any positive integer value from 2 to l = any positive integer value from 2 to n + 1 m l = any integer...
-
Does log 81 (2401) = log 3 (7)? Verify the claim algebraically.
-
The bond length in C2 is 131 pm. Compare this with the bond lengths in C2H2 (120 pm), C2H4 (134 pm), and C2H6 (153 pm). What bond order would you predict for C2 from its bond length? Does this agree...
-
Draw resonance formulas of the nitric acid molecule, HNO3. What is the geometry about the N atom? What is the hybridization on N? Use bond energies and one Lewis formula for HNO3 to estimate Hf for...
-
One resonance formula of benzene, C6H6, is What is the other resonance formula? What is the geometry about a carbon atom? What hybridization would be used in valence bond theory to describe the...
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...

Study smarter with the SolutionInn App


