Question: Using the following reaction (depicted using molecular models), large quantities of ammonia are burned in the presence of a platinum catalyst to give nitric oxide,
Using the following reaction (depicted using molecular models), large quantities of ammonia are burned in the presence of a platinum catalyst to give nitric oxide, as the first step in the preparation of nitric acid.
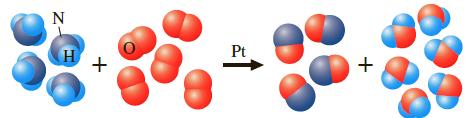
Suppose a vessel contains 5.5 g of NH3. How many grams of O2 are needed for a complete reaction?
N H Pt +
Step by Step Solution
3.48 Rating (164 Votes )
There are 3 Steps involved in it
From the molecular models the ... View full answer

Get step-by-step solutions from verified subject matter experts


