Question: Using the ionic radii given in Table 9.3, estimate the energy to form a mole of Na + F - ion pairs from the corresponding
Using the ionic radii given in Table 9.3, estimate the energy to form a mole of Na+F- ion pairs from the corresponding atomic ions.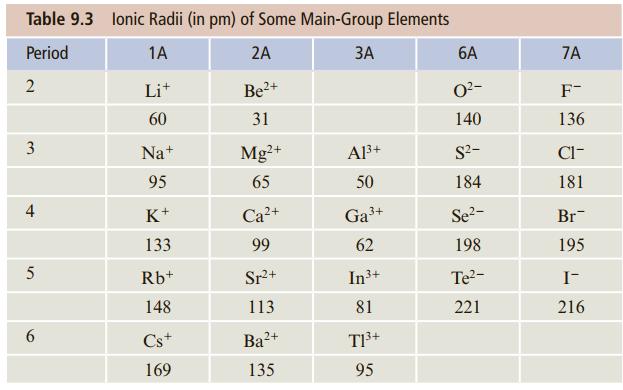
Table 9.3 lonic Radii (in pm) of Some Main-Group Elements Period 1A 2A 6A 7A 2 Lit 2+ O?- F- 60 31 140 136 3 Na+ Mg+ Al3+ S?- Cl- 95 65 50 184 181 4 K+ + Ga3+ Se2- Br- 133 99 62 198 195 Rb+ Sr+ In3+ Te?- I- 148 113 81 221 216 6. Cs+ Ba?+ T3+ 169 135 95
Step by Step Solution
3.30 Rating (171 Votes )
There are 3 Steps involved in it
r Na 95 pm r F 136 pm d ion pair r Na r F 95 pm 136 pm ... View full answer

Get step-by-step solutions from verified subject matter experts


