What hybrid orbitals would be expected for the central atom in each of the following molecules or
Question:
What hybrid orbitals would be expected for the central atom in each of the following molecules or ions?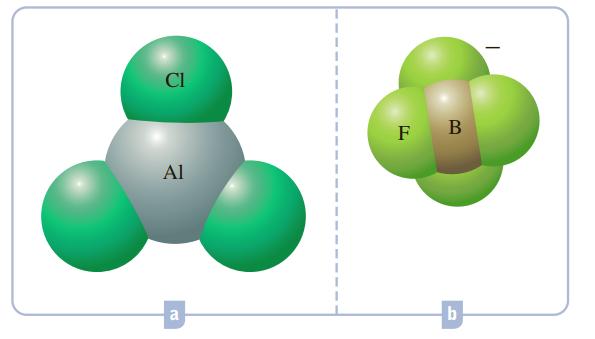
Transcribed Image Text:
CI F B Al a
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
a AlCl 3 is a trigonal planar ...View the full answer

Answered By

Dorcas Juliet
I am a proficient tutor and writer with over 4 years experience, I can deliver A+ works in all fields related to business and economics subject. Kindly hire me for excellent papers
4.70+
10+ Reviews
51+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the following molecules or ions of sulfur and oxygen, write a single Lewis structure that obeys the octet rule, and calculate the oxidation numbers and formal charges on all the atoms:...
-
Predict the geometry for the central atom in each of the compounds below: a) NH 3 b) H 3 O + c) BH 4 d) BCl 3 e) BCl 4 f) CCl 4 g) CHCl 3 h) CH 2 Cl 2
-
What F 2 phenotypic segregation ratio would be expected for the cross described in the preceding problem if the dominant allele, C, of the third gene produced a product that completely inhibited the...
-
Let a 0. Solve |x| = 3.
-
Explain how a swaption can be terminated at expiration by either exercising it or settling it in cash. Why are these procedures financially equivalent?
-
Permeability of sandstone during weathering. Natural stone, such as sandstone, is a popular building construction material. An experiment was carried out in order to better understand the decay...
-
Web-based exercise. If you did any of Exercises 13.21 to 13.30, check your calculations using the Normal Curve applet described in the previous exercise.
-
Zucca Associates, a law firm, hires Attorney Odessa Smythe at an annual salary of $ 192,000. The law firm expects her to spend 2,400 hours per year performing legal work for clients. Indirect costs...
-
Question Write a reflection which highlights two of the most important lessons you have learnt in the course Accounting & Finance. Additionally, explain how what you have learnt will benefit the...
-
Kijiji Auctions runs an online auction company. Its end-of-year financial statements indicate the following results. Calculate the companys net profit margin expressed as a percent (to one decimal...
-
What hybrid orbitals would be expected for the central atom in each of the following molecules or ions? a. SeI 2 b. NO 3 - c. BeCl 2 d. ClO 4 -
-
What hybrid orbitals would be expected for the central atom in each of the following molecules or ions? Cl F a b.
-
On September 2, 2011, Levine executed a mortgage bond under which she promised to pay the Mykoffs a preexisting obligation of $54,000. On October 14, 2017, the Mykoffs transferred the mortgage to...
-
1. How will you check if a class is a child of another class? 2. What is init method in python?
-
1. What are lists and tuples? What is the key difference between the two? 2. What is Scope in Python?
-
1. What is an Interpreted language? 2. What is a dynamically typed language?
-
Q.1 If denotes increasing order of intensity, then the meaning of the words [talk shout scream] is analogous to [please pander]. Which one of the given options is appropriate to fill the blank? (A)...
-
Write the complete ground-state electron configuration of the strontium atom, Sr, using the building-up principle.
-
Audrey purchases a riding lawnmower using a 2-year, no-interest deferred payment plan at Lawn Depot for x dollars. There was a down payment of d dollars and a monthly payment of m dollars. Express...
-
a. Draw Lewis structures of each of the following compounds: LiH, NH3, CH4, CO2. b. Which of these has the highest boiling point? Why? c. Which of these has the lowest boiling point? Why? d. Which of...
-
Describe the contents of a carbon dioxide fire extinguisher at 20C. Then describe it at 35C. Explain the difference.
-
Discuss why supercritical carbon dioxide is a nearly ideal solvent.
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


