What hybrid orbitals would be expected for the central atom in each of the following molecules or
Question:
What hybrid orbitals would be expected for the central atom in each of the following molecules or ions?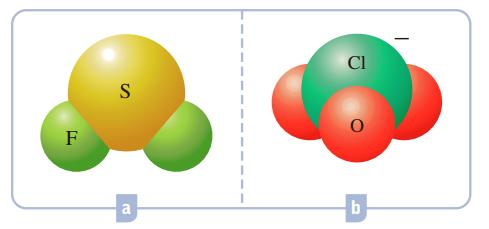
Transcribed Image Text:
Cl F a b.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
a SF 2 is a bent molecule The ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the following molecules or ions of sulfur and oxygen, write a single Lewis structure that obeys the octet rule, and calculate the oxidation numbers and formal charges on all the atoms:...
-
Predict the geometry for the central atom in each of the compounds below: a) NH 3 b) H 3 O + c) BH 4 d) BCl 3 e) BCl 4 f) CCl 4 g) CHCl 3 h) CH 2 Cl 2
-
What F 2 phenotypic segregation ratio would be expected for the cross described in the preceding problem if the dominant allele, C, of the third gene produced a product that completely inhibited the...
-
The exact perimeter P of a square is 50 feet. What measured lengths are possible for the side S of the square to have relative error in the perimeter that is less than or equal to 0.04 (or 4%)?
-
A company wants to enter into a commitment to initiate a swap in 90 days. The swap would consist of four payments 90 days apart with the underlying being LIBOR. Use the following term structure of...
-
Symmetric or skewed? Would you expect the data sets that follow to possess relative frequency distributions that are symmetric, skewed to the right, or skewed to the left? Explain. a. The salaries of...
-
Describe the difference between functional and psychological needs.
-
What marketing recommendations, including pricing recommendations, would you make to Southwest as it moves into the next decade? Its the same plane going to the same place at exactly the same time....
-
On January 1, 2017, the Hardin Company budget committee has reached agreement on the following data for the 6 months ending June 30, 2017. Sales units: First quarter 5,200; second quarter 6,700;...
-
Determine whether f is a function from the set of all bit strings to the set of integers if a) f (S) is the position of a 0 bit in S. b) f (S) is the number of 1 bits in S. c) f (S) is the smallest...
-
What hybrid orbitals would be expected for the central atom in each of the following molecules or ions? CI F B Al a
-
Which of the following molecules would be expected to have a dipole moment of zero because of symmetry? a. BeBr 2 b. H 2 Se c. AsF 3 d. SeF 6
-
What are the Quality Records (QRs) and work products of SITE?
-
What are the elements of partnership ?
-
1. (2x+3)dx
-
Write the complete ground-state electron configuration of the tellurium atom, Te, using the building-up principle.
-
What are conversion costs? What are prime costs?
-
A geckos toes have been shown to stick to walls through van der Waals forces. Van der Waals forces also exist between your finger and a wall. Why, then, doesnt your finger stick to the wall in the...
-
Although a geckos toes stick easily to a wall, their toes lift off a surface just as easily. Explain.
-
Describe the structure of a nematic liquid crystal. How is it similar to a liquid? How is it similar to a crystalline solid?
-
Dr. Claudia Gomez, a plastic surgeon, had just returned from a conference in which she learned of a new surgical procedure for removing wrinkles around eyes, reducing the time to perform the normal...
-
QUESTION 2 ( 2 0 Marks ) 2 . 1 REQUIRED Study the information provided below and prepare the Income Statement for the year ended 3 1 December 2 0 2 3 using the marginal costing method. INFORMATION...
-
DROP DOWN OPTIONS: FIRST SECOND THIRD FOURTH 5. Cost of new common stock A firm needs to take flotation costs into account when it is raising capital fromY True or False: The following statement...

Study smarter with the SolutionInn App


