A flat plate of solid carbon is being burned in the presence of pure oxygen according to
Question:
A flat plate of solid carbon is being burned in the presence of pure oxygen according to the reaction
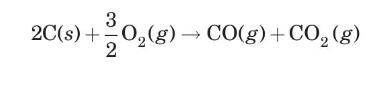
Molecular diffusion of gaseous reactant and products takes place through a gas film adjacent to the carbon surface; the thickness of this film is \(1.0 \mathrm{~mm}\). On the outside of the film, the gas concentration is \(40 \% \mathrm{CO}, 20 \% \mathrm{O}_{2}\), and \(40 \% \mathrm{CO}_{2}\). The reaction at the surface may be assumed to be instantaneous; therefore, next to the carbon surface, there is virtually no oxygen. The temperature of the gas film is \(600 \mathrm{~K}\), and the pressure is 1 bar. Estimate the rate of combustion of the carbon, in \(\mathrm{kg} / \mathrm{m}^{2}-\mathrm{min}\), and the interface concentration.
Step by Step Answer:






