Calculate the thermal conductivity of air, hydrogen, and carbon dioxide at (300 mathrm{~K}), assuming ideal gas behavior.
Question:
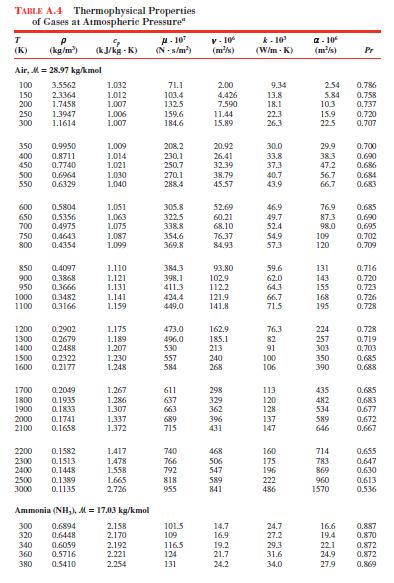
Calculate the thermal conductivity of air, hydrogen, and carbon dioxide at \(300 \mathrm{~K}\), assuming ideal gas behavior. Compare your calculated values to values from Table A.4.
Data From Table A.4:-
Transcribed Image Text:
TABLE A.4 Thermophysical Properties of Gases at Atmospheric Pressure" T (K) P (kg/m) (kJ/kg- A-10 V-10 (kJ/kg -K) (N-5/m) (m/s) k-10' (W/m-K) -10 (m/s) Pr Air, M=28.97 kg/kmol 100 3.5562 1.032 71.1 2.00 9.34 2.54 0.786 150 2.3364 1.012 103.4 4.426 13.8 5.84 0.758 200 1.7458 1.007 132.5 7.590 18.1 10.3 0.737 250 1.3947 1.006 159.6 11.44 22.3 15.9 0.720 300 1.1614 1.007 184.6 15.89 26.3 22.5 0.707 350 0.9950 1.009 208.2 20.92 30.0 29.9 0.700 400 0.8711 1.014 230.11 26.41 33.8 38.3 0.690 450 0.7740 1.021 250.7 32.39 37.3 47.2 0.686 500 0.6964 1.030 270.1 38.79 40.7 56.7 0.684 550 0.6329 1.040 288.4 45.57 43.91 66.7 0.683 600 0.5804 1.051 305.8 52.69 46.9 76.9 0.685 650 0.5356 1.063 322.5 60.21 49.7 873 0.690 700 0.4975 1.075 338.8 68.10 52.4 98.0 0.695 750 0.4643 1.087 354.6 76.37 54.9 109 0.702 800 0.4354 1.099 369.8 84.93 57.3 120 0.709 850 0.4097 1.110 384.3 93.80 59.6 131 0.716 900 0.3868 1.121 398.1 102.9 62.0 143 0.720 950 0.3666 1.131 411.3 112.2 64.3 155 0.723 1000 0.3482 1.141 424.4 121.9 66.7 168 0.726 1100 0.3166 1.159 449.0 141.8 71.5 195 0.728 1200 0.2902 1.175 473.0 162.9 76.3 224 0.728 1300 0.2679 1.189 496.0 185.1 82 257 0.719 1400 0.2488 1.207 530 213 91 303 0.703 1500 0.2322 1.230 557 240 100 350 0.685 1600 0.2177 1.248 584 268 106 390 0.688 1700 0.2049 1.267 611 298 113 435 0.685 1800 0.1935 1.286 637 329 120 482 0.683 1900 0.1833 1.307 663 362 128 534 0.677 2000 0.1741 1.337 689 396 137 589 0.672 2100 0.1658 1.372 715 431 147 646 0.667 2200 0.1582 1.417 740 468 160 714 0.655 2300 0.1513 1.478 766 506 175 783 0.647 2400 0.1448 1.558 792 547 196 869 0.630 2500 0.1389 1.665 818 589 222 960 0.613 3000 0.1135 2726 955 841 486 1570 0.536 Ammonia (NH,), M = 17.03 kg/kmol 300 0.6894 2.158 101.5 14.7 24.7 16.6 0.887 320 0.6448 2.170 109 16.9 27.2 19.4 0.870 340 0.6059 2.192 116.5 19.2 29.3 22.1 0.872 360 0.5716 2.221 124 21.7 31.6 24.9 0.872 380 0.5410 2.254 131 24.2 34.0 27.9 0.869
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

John Kago
Am a processional practicing accountant with 5 years experience in practice, I also happens to have hands on experience in economic analysis and statistical research for 3 years. am well conversant with Accounting packages, sage, pastel, quick books, hansa world, etc, I have real work experience with Strata, and SPSS
4.70+
31+ Reviews
77+ Question Solved
Related Book For 

Fundamentals Of Heat And Mass Transfer
ISBN: 9781119220442
8th Edition
Authors: Theodore L. Bergman, Adrienne S. Lavine
Question Posted:
Students also viewed these Engineering questions
-
Calculate the thermal conductivity of air, hydrogen, and carbon dioxide at 300 K, assuming ideal gas behavior. Compare your calculated values to values from Table A.4.
-
Compare the Investment Criteria by considering the following two mutually exclusive projects: Year..Cash flow (A) ..Cash flow (B) 0..-350,000-50,000 1..45,00024,000 2..65,00022,000...
-
(a) Advanced Computer Sdn Bhd is involved in the manufacturing of computer network equipment. For the next month, the company estimated to make sales of 250 units computer network equipment totaling...
-
Consider the examples of groupthink explained in this chapter and discuss how they can relate to situations you have experienced on your projects.
-
Three individuals organized Pest Away Corporation on January 1, 2012, to provide insect extermination services. The company paid dividends of $10,000 during the year. At the end of 2012, the...
-
Why might airline managers choose to lease rather than purchase their planes?
-
The financial statements of Danube Engineering plc for the year that has just ended are as follows: Income statement for the year ending 31 March Year 5 m Sales revenue 500 Cost of sales ( 350 )...
-
The following disclosure note appeared in the December 26, 2015, annual report of the Intel Corporation. Note 5: Cash and Investments (partial) Available-for-sale investments as of December 26, 2015,...
-
astrid transport es coral basis account Tooming transactions Eerdee-42 (Algorithe) Revenue and Recognition Astrid Transportation Inc, headquartered in Atlanta, Georgia, ied customers $2,309,490 for...
-
The thermal conductivity of helium at a certain temperature is \(0.15 \mathrm{~W} / \mathrm{m} \cdot \mathrm{K}\). Calculate the helium temperature assuming ideal gas behavior, and compare it to the...
-
Consider a \(400 \mathrm{~mm} \times 400 \mathrm{~mm}\) window in an aircraft. For a temperature difference of \(90^{\circ} \mathrm{C}\) from the inner to the outer surface of the window, calculate...
-
Does it matter in which order accounts are listed in the trial balance?
-
Go to: https://www.instagram.com/ryderseyewear/ on your desktop, laptop, or mobile (or a combination of all 3). You are the new Social Media Marketing Manager for Ryders Eyewear. You've been asked...
-
As leaders, it is very important that we have the ability to assess our own motivation and the motivation of others around us. It is also important to recognize the key factors involved in...
-
At the end of this exam, you will find Article 1 - " How Companies Can Prepare for a Long Run of High Inflation ". Please read the article and, when necessary, consult additional sources and the...
-
You can develop your capabilities as a manger by better understanding different ways of motivating and rewarding employees. You can also better prepare for your own career by better understanding the...
-
Topic: Project Malasakit of Kara David https://projectmalasakit.org/ What is the pros and cons of these alternative courses of the action below: Strengthen the internal organization via promoting it...
-
In a political science class of 35 students, 21 favor abolishing the Electoral College and thus electing the President of the United States by popular vote. If two students are selected at random...
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


