The thermal conductivity of helium at a certain temperature is (0.15 mathrm{~W} / mathrm{m} cdot mathrm{K}). Calculate
Question:
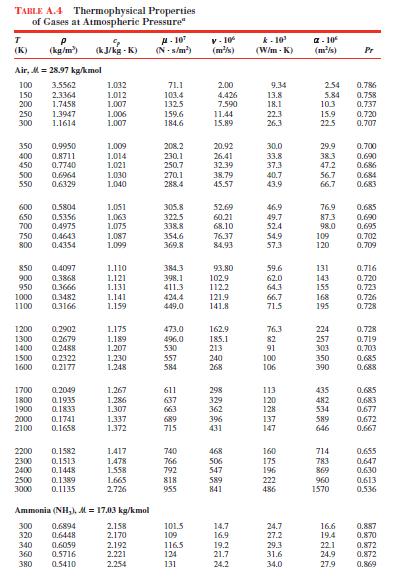
The thermal conductivity of helium at a certain temperature is \(0.15 \mathrm{~W} / \mathrm{m} \cdot \mathrm{K}\). Calculate the helium temperature assuming ideal gas behavior, and compare it to the temperature found in Table A.4.
Data From Table A.4:-
Transcribed Image Text:
TABLE A.4 Thermophysical Properties of Gases at Atmospheric Pressure" T (K) P (kg/m) (kJ/kg- A-10 V-10 (kJ/kg -K) (N-5/m) (m/s) k-10' (W/m-K) -10 (m/s) Pr Air, M=28.97 kg/kmol 100 3.5562 1.032 71.1 2.00 9.34 2.54 0.786 150 2.3364 1.012 103.4 4.426 13.8 5.84 0.758 200 1.7458 1.007 132.5 7.590 18.1 10.3 0.737 250 1.3947 1.006 159.6 11.44 22.3 15.9 0.720 300 1.1614 1.007 184.6 15.89 26.3 22.5 0.707 350 0.9950 1.009 208.2 20.92 30.0 29.9 0.700 400 0.8711 1.014 230.11 26.41 33.8 38.3 0.690 450 0.7740 1.021 250.7 32.39 37.3 47.2 0.686 500 0.6964 1.030 270.1 38.79 40.7 56.7 0.684 550 0.6329 1.040 288.4 45.57 43.91 66.7 0.683 600 0.5804 1.051 305.8 52.69 46.9 76.9 0.685 650 0.5356 1.063 322.5 60.21 49.7 873 0.690 700 0.4975 1.075 338.8 68.10 52.4 98.0 0.695 750 0.4643 1.087 354.6 76.37 54.9 109 0.702 800 0.4354 1.099 369.8 84.93 57.3 120 0.709 850 0.4097 1.110 384.3 93.80 59.6 131 0.716 900 0.3868 1.121 398.1 102.9 62.0 143 0.720 950 0.3666 1.131 411.3 112.2 64.3 155 0.723 1000 0.3482 1.141 424.4 121.9 66.7 168 0.726 1100 0.3166 1.159 449.0 141.8 71.5 195 0.728 1200 0.2902 1.175 473.0 162.9 76.3 224 0.728 1300 0.2679 1.189 496.0 185.1 82 257 0.719 1400 0.2488 1.207 530 213 91 303 0.703 1500 0.2322 1.230 557 240 100 350 0.685 1600 0.2177 1.248 584 268 106 390 0.688 1700 0.2049 1.267 611 298 113 435 0.685 1800 0.1935 1.286 637 329 120 482 0.683 1900 0.1833 1.307 663 362 128 534 0.677 2000 0.1741 1.337 689 396 137 589 0.672 2100 0.1658 1.372 715 431 147 646 0.667 2200 0.1582 1.417 740 468 160 714 0.655 2300 0.1513 1.478 766 506 175 783 0.647 2400 0.1448 1.558 792 547 196 869 0.630 2500 0.1389 1.665 818 589 222 960 0.613 3000 0.1135 2726 955 841 486 1570 0.536 Ammonia (NH,), M = 17.03 kg/kmol 300 0.6894 2.158 101.5 14.7 24.7 16.6 0.887 320 0.6448 2.170 109 16.9 27.2 19.4 0.870 340 0.6059 2.192 116.5 19.2 29.3 22.1 0.872 360 0.5716 2.221 124 21.7 31.6 24.9 0.872 380 0.5410 2.254 131 24.2 34.0 27.9 0.869
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Fundamentals Of Heat And Mass Transfer
ISBN: 9781119220442
8th Edition
Authors: Theodore L. Bergman, Adrienne S. Lavine
Question Posted:
Students also viewed these Engineering questions
-
(a) Advanced Computer Sdn Bhd is involved in the manufacturing of computer network equipment. For the next month, the company estimated to make sales of 250 units computer network equipment totaling...
-
1. A financial institution owns a portfolio of options dependent on the US dollar-sterling exchange rate. The delta of the portfolio with respect to percentage changes in the exchange rate is 6.1. If...
-
Compare the Investment Criteria by considering the following two mutually exclusive projects: Year..Cash flow (A) ..Cash flow (B) 0..-350,000-50,000 1..45,00024,000 2..65,00022,000...
-
Explain how you solve problems during the implementation of your projects. Does this operate as a formal approach and/or use specific supportive techniques?
-
DSW, Inc., is a designer shoe warehouse, selling some of the most luxurious and fashionable shoes at prices that people can actually afford. Its balance sheet, at January 29, 2011, contained the...
-
Why is the layout of warehouses important? Supermarkets are really one type of warehouse, so the same factors will be important in each. Is this true?
-
Changes Ltd owns five shops selling fashion goods. In the past the business maintained a healthy cash balance. However, this has fallen in recent months and at the end of September Year 10 the...
-
Prepare the 2015 statement of cash flows for Smolira Golf Corp. Some recent financial statements for Smolira Golf Corp. follow. SMOLIRA GOLF CORP 2014 and 2015 Balance Sheets Assets Liabilities and...
-
Current Attempt in Progress Skysong Company purchased a new machine on September 1, 2022, at a cost of $125,000. The company estimated that the machine will have a salvage value of $12,000. The...
-
A method for determining the thermal conductivity \(k\) and the specific heat \(c_{p}\) of a material is illustrated in the sketch. Initially the two identical samples of diameter \(D=50...
-
Calculate the thermal conductivity of air, hydrogen, and carbon dioxide at \(300 \mathrm{~K}\), assuming ideal gas behavior. Compare your calculated values to values from Table A.4. Data From Table...
-
The D.J. Masson Corporation needs to raise $500,000 for 1 year to supply working capital to a new store. Masson buys from its suppliers on terms of 3/10, net 90, and it currently pays on the 10th day...
-
In the introduction to "The Five Sexes," Anne Fausto-Sterling writes that she had to "invent conventions - s/he and his/her - to denote someone who is clearly neither male nor female or who is...
-
Select a product described as one of the "Biggest Product Flops" of 2019 that you will bring back to the market. To, you will need to engage in some research to understand why the product failed to...
-
Breaking the Bank Case Questions (video found at: http://www.pbs.org/wgbh/pages/frontline/breakingthebank/view/?utm_campaign=viewpage &utm_medium=grid&utm_source=grid) 1) To what extent were the...
-
Please answer in full and write legibly. Suppose Alice has taken 7 classes college, and her current GPA is 3.48 (assume for simplicity that all courses carry the same number of credits). Answer the...
-
F. Explain how to overcome two potential biases (e.g., prejudice, discrimination) using culturally competent strategies that will help improve stakeholder communication. G. Explain how to mitigate...
-
Forty-seven employees in an office wear eyeglasses. Thirty-one have single-vision correction, and 16 wear bifocals. If two employees are selected at random from this group, what is the probability...
-
1) Predict the organicproduct formed when BzCl reacts with cyclohexanol. BzCl = benzoylchloride. 2) Provide the majororganic product of the reaction below. 3) Draw the structureof the product formed...
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


