Consider a nickel wall separating hydrogen gas that is maintained on one side at 5 atm and
Question:
Consider a nickel wall separating hydrogen gas that is maintained on one side at 5 atm and on the opposite at 3 atm. If the temperature is constant at 85°C, determine
(a) The mass densities of hydrogen gas in the nickel wall on both sides
(b) The mass densities of hydrogen outside the nickel wall on both sides.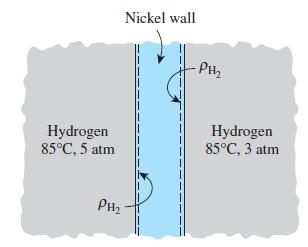
Transcribed Image Text:
Hydrogen 85°C, 5 atm PH₂ Nickel wall I 1 -PH₂2₂ Hydrogen 85°C, 3 atm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
To determine the mass densities of hydrogen gas in the nickel wall on both sides we can use the following equation nV M where is the mass density kgm ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Heat And Mass Transfer Fundamentals And Applications
ISBN: 9780073398181
5th Edition
Authors: Yunus Cengel, Afshin Ghajar
Question Posted:
Students also viewed these Engineering questions
-
Consider a rubber membrane separating carbon dioxide gas that is maintained on one side at 2 atm and on the opposite at 1 atm. If the temperature is constant at 25C, determine (a) The molar densities...
-
Hydrogen gas at 85C is maintained at constant pressures of 5 atm and 3 atm on opposite sides of a 0.1-mm-thick nickel wall. Determine the molar diffusion rate per unit area through the nickel wall....
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
How are the interface and implementation sections of an Objective-C class specified?
-
At October 31, Prasad Company made an accrued expense adjusting entry of $1,680 for salaries. Prepare the reversing entry on November 1, and indicate the balances in Salaries and Wages Payable and...
-
Why might someone use the corporate model even though the dividend model could be used? AppendixLO1
-
Develop a model of how you think the warehouse should work in this environment. LO.1
-
In what way is foreign currency translation tied to foreign inflation?
-
Credit A trial balance before adjustment included the following: Debit Accounts receivable $120,000 Allowance for doubtful accounts Sales Sales retums and allowances 8,000 730 $S10,000 Give journal...
-
Ozuna Company uses a job-order costing system with a plantwide predetermined overhead rate based on direct labor-hours. For job costing purposes, it uses an average direct labor wage rate of $20 per...
-
Pure nitrogen gas at 200 kPa and 25C is flowing inside a long circular rubber pipe. The atmospheric air surrounding the pipe is at 1 atm and 25C, with 79 percent nitrogen and 21 percent oxygen....
-
Hydrogen gas at 750 kPa and 85C is stored in a spherical nickel vessel. The vessel is situated in a surrounding of atmospheric air at 1 atm. Determine the molar and mass concentrations of hydrogen in...
-
CodeDeskInc matches programmers with freelance jobs. It has 30 employees who staff its online chat room. It receives, on average, 240 chat requests per hour, and the average chat session takes 5...
-
The four classic leadership styles There are four leadership styles which are prominent in today's businesses and companies. They are Laissez-faire, Autocratic, Democratic, and Charismatic...
-
How can conflict be viewed positively? Cite a specific example of when this might be the case. Under what circumstances might "avoiding" conflict be "managing" conflict? In other words, when might...
-
Visit the website and answer the questions below. https://www.forbes.com/advisor/business/software/best-crm-small-business/ Based on the CRM software discussed in the article, which 3 software...
-
Planning consists of translating and organizations mission and vision into objectives. The organization's purpose is expressed as a mission statement, and what it becomes is expressed as a vision...
-
Question 1- Visit the Boots and Hearts Festival website: www.bootsandhearts.com. Using the information you find on the site, make an analysis of the festival's Strengths, Weaknesses, Opportunities...
-
Consolidations of Financial Statement, bases on research Wal-Mart Stores, explain the corporate structure in terms of consolidation. How is it organized from a consolidated viewpoint? What are the...
-
Could a set of three vectors in span all of? Explain. What about n vectors in when n is less than m? R4
-
What is the exact relationship between the static error constants and the steady-state errors for ramp and parabolic inputs?
-
The steady-state error in velocity of a system is defined to be where r is the system input, and c is the system output. Find the steady-state error in velocity for an input of t 3 u(t) to a unity...
-
For a step input, the steady-state error is approximately the reciprocal of the static error constant if what condition holds true?
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


