Consider the nicotine extraction of Problems 3.21 and 3.22. Calculate the number of ideal stages required to
Question:
Consider the nicotine extraction of Problems 3.21 and 3.22. Calculate the number of ideal stages required to achieve at least $95 \%$ extraction efficiency.
Data From Problem 3.21:-
Nicotine in a water solution containing $2 \%$ nicotine is to be extracted with kerosene at $293 \mathrm{~K}$. Water and kerosene are essentially insoluble in each other. Determine the percentage extraction of nicotine if $100 \mathrm{~kg}$ of the feed solution is extracted in a sequence of four batch ideal extractions using $49.0 \mathrm{~kg}$ fresh, pure kerosene each. The equilibrium data are given as follows (Claffey et al., 1950):
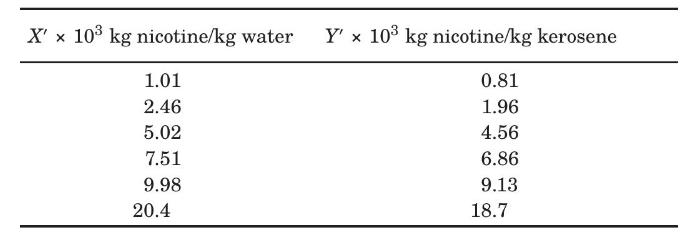
Data From Problem 3.22:-
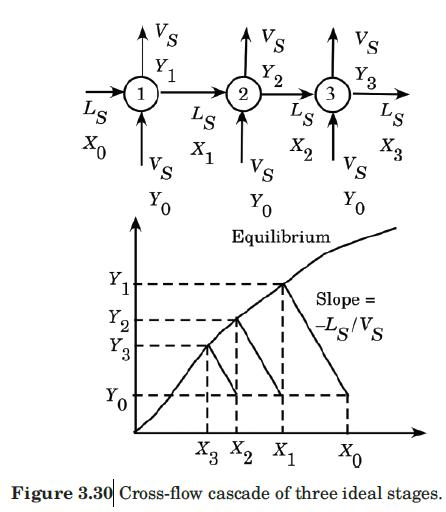
The drying and liquid-liquid extraction operations described in Problems 3.20 and 3.21, respectively, are examples of a flow configuration called a cross-flow cascade. Figure 3.30 is a schematic diagram of a cross-flow cascade of ideal stages. Each stage is represented by a circle, and within each stage mass transfer occurs as if in cocurrent flow. The $L$ phase flows from one stage to the next, being contacted in each stage by a fresh $V$ phase. If the equilibrium-distribution curve of the cross-flow cascade is everywhere straight and of slope $m$, it can be shown that (Treybal, 1980)
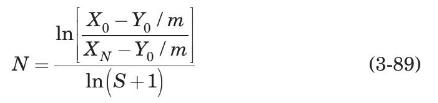
where $S$ is the stripping factor, $m V_{S} / L_{S}$, constant for all stages, and $N$ is the total number of stages. Solve Problem (3.21) using equation (3-89) and compare the results obtained by both methods.
Figure 3.30:-
Data From Problem 3.21:-
Nicotine in a water solution containing $2 \%$ nicotine is to be extracted with kerosene at $293 \mathrm{~K}$. Water and kerosene are essentially insoluble in each other. Determine the percentage extraction of nicotine if $100 \mathrm{~kg}$ of the feed solution is extracted in a sequence of four batch ideal extractions using $49.0 \mathrm{~kg}$ fresh, pure kerosene each. The equilibrium data are given as follows (Claffey et al., 1950):

Step by Step Answer:






