Nicotine in a water solution containing $2 %$ nicotine is to be extracted with kerosene at $293
Question:
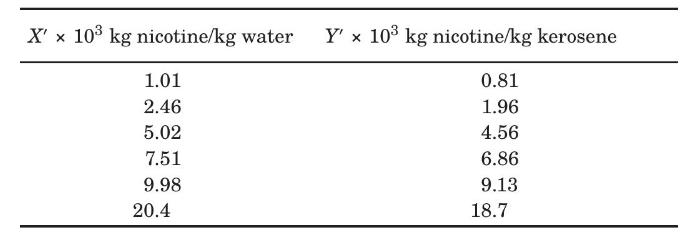
Nicotine in a water solution containing $2 \%$ nicotine is to be extracted with kerosene at $293 \mathrm{~K}$. Water and kerosene are essentially insoluble in each other. Determine the percentage extraction of nicotine if $100 \mathrm{~kg}$ of the feed solution is extracted in a sequence of four batch ideal extractions using $49.0 \mathrm{~kg}$ fresh, pure kerosene each. The equilibrium data are given as follows (Claffey et al., 1950):
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





