If one or both components of a binary gas mixture are polar, a modified Lennard-Jones relation is
Question:
If one or both components of a binary gas mixture are polar, a modified Lennard-Jones relation is often used. Brokaw (equation (1969) has suggested an alternative method for this case. 1-49) is used, but the collision integral is now given by
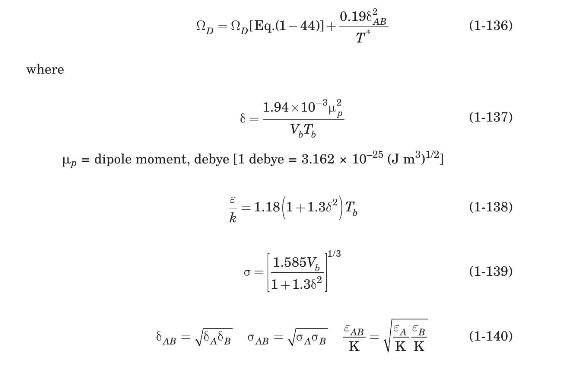
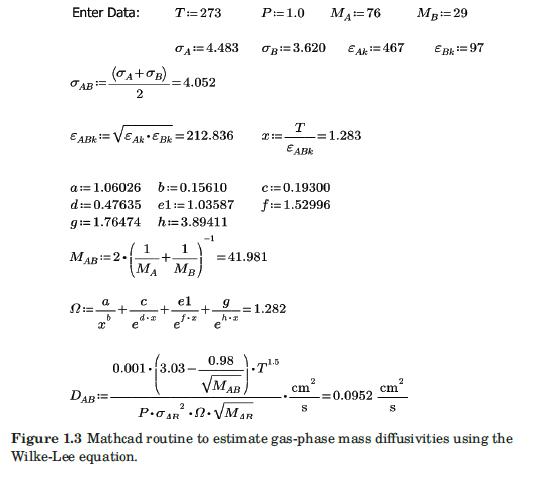
(a) Modify the Mathcad routine of Figure 1.3 to implement Brokaw's method.
(b) Estimate the diffusion coefficient for a mixture of methyl chloride and sulfur dioxide at 1 bar and \(323 \mathrm{~K}\), and compare it to the experimental value of \(0.078 \mathrm{~cm}^{2} / \mathrm{s}\). The data required to use Brokaw's relation are shown below (Reid et al., 1987):
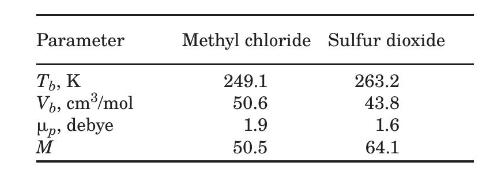
Data From Figure 1.3:-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





