It is desired to adsorb (99.8 %) of the toluene originally present in the waste gas of
Question:
It is desired to adsorb \(99.8 \%\) of the toluene originally present in the waste gas of Problem 1.10. Estimate how much activated carbon should be used if the system is allowed to reach equilibrium at constant temperature and pressure.
Data From Problem 1.10:-
A waste gas contains \(0.5 \%\) toluene in air and occupies a volume of \(2500 \mathrm{~m}^{3}\) at \(298 \mathrm{~K}\) and \(101.3 \mathrm{kPa}\). To reduce the toluene content of this gas, it is exposed to \(100 \mathrm{~kg}\) of activated carbon, initially free of toluene. The system is allowed to reach equilibrium at constant temperature and pressure.
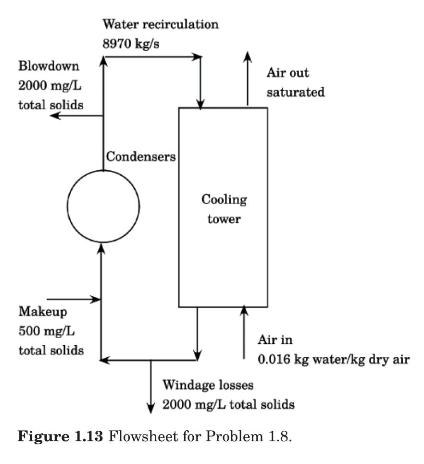
If the air does not adsorb on the carbon, calculate the equilibrium concentration of toluene in the gaseous phase, and the amount of toluene adsorbed by the carbon. The adsorption equilibrium for this system is given by the Freundlich isotherm (USEPA, 1987):
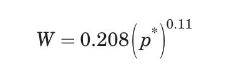
where \(W\) is the carbon equilibrium adsorptivity, in \(\mathrm{kg}\) of toluene/kg of carbon, and \(p^{*}\) is the equilibrium toluene partial pressure, in \(\mathrm{Pa}\), and must be between 0.7 and \(345 \mathrm{~Pa}\).
Step by Step Answer:






