(a) Describe the bonding in the M 2 diatomics (M = Li, Na, K, Rb, Cs) in...
Question:
(a) Describe the bonding in the M2 diatomics (M = Li, Na, K, Rb, Cs) in terms of valence bond and molecular orbital theories.
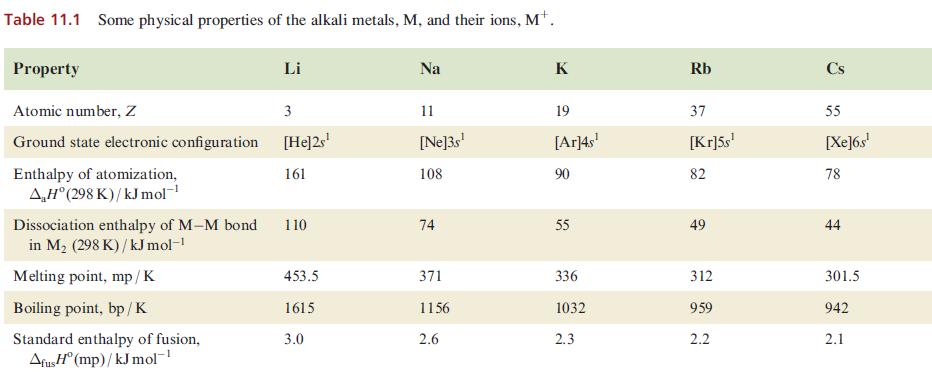
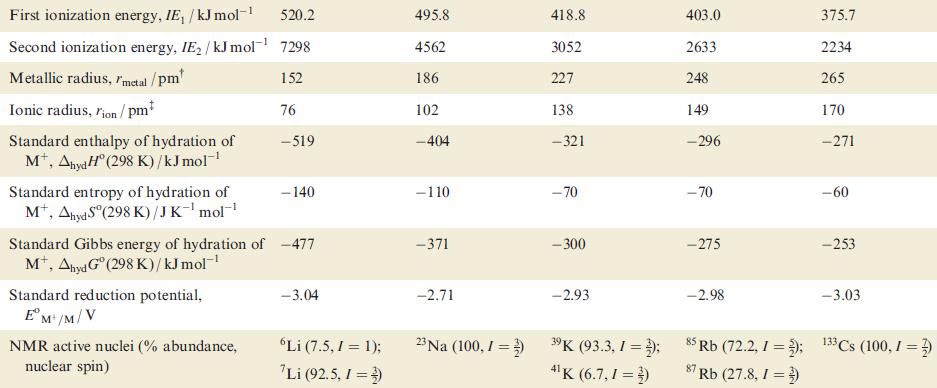
(b) Account for the trend in metal–metal bond dissociation energies given in Table 11.1.
Transcribed Image Text:
Table 11.1 Some physical properties of the alkali metals, M, and their ions, Mt. Property Atomic number, Z Ground state electronic configuration [He]2s¹ 161 Enthalpy of atomization, AH°(298 K)/kJ mol-¹ Dissociation enthalpy of M-M bond in M₂ (298 K)/kJ mol-¹ Melting point, mp/K Li Boiling point, bp/K Standard enthalpy of fusion, Afus H (mp)/kJ mol-¹ 3 110 453.5 1615 3.0 Na 11 [Ne]3s¹ 108 74 371 1156 2.6 K 19 [Ar]4s¹ 90 55 336 1032 2.3 Rb 37 [Kr]5s¹ 82 49 312 959 2.2 Cs 55 [Xe]6s¹ 78 44 301.5 942 2.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a Bonding in M2 diatomics M Li Na K Rb Cs in terms of valence bond and molecular orbital theories Valence Bond Theory In valence bond theory chemical bonds are formed by the overlap of atomic orbitals ...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the Divergence Test to determine whether the following series diverge or state that the test is inconclusive. k k=1 k2 + 1
-
What is the quadratic formula? Start with x=
-
Quantity A Quantity B -(x - 3)/4 y a. Quantity A is greater. b. Quantity B is greater. c. The two quantities are equal. d. The relationship...
-
If we define the median of a sequence as a number so that exactly as many elements come before it in the sequence as come after it, fix the program in 4.6.3 so that it always prints out a median....
-
What is the key feature of activity-based responsibility accounting? Briefly explain.
-
Find all points (x, y) where f (x, y) has a possible relative maximum or minimum. Then, use the second-derivative test to determine, if possible, the nature of f (x, y) at each of these points. If...
-
2. Using the BinomCall and BinomPut functions, compute the binomial approximations for the options in Examples 1 and 2. Be sure to compute prices for n = 8, 9, 10, 11, and 12. What do you observe...
-
Incremental analysis involves the accumulation of information concerning a single course of action. Do you agree? Why?
-
BE17-13 (similar to) Likeness Mirrors, Ltd. offers a 3-year warranty on all its products In Year 1, the company reported income before warranty expense of 5630,000 and estimated that warranty repas...
-
Magnesium hydride possesses a rutile lattice. (a) Sketch a unit cell of rutile. (b) What are the coordination numbers and geometries of the Mg and H centres in this structure?
-
Write equations for the following processes, noting appropriate conditions: (a) Electrolysis of water; (b) Electrolysis of molten LiH; (c) CaH 2 reacting with water; (d) Mg treated with dilute nitric...
-
What is the critical delay path in the multiplier in Figure 3.35? What is the delay along this path in terms of the number of gates? m3 FA P7 P6 m3 m FA FA P5 m3 m m FA FA P4 O FA m mo m2 D- FA FA P3...
-
Adidas-Consumer Goods STEP ONE: MISSION: Mission statement core message that guides and influences your marketing strategy. Why is this company in business and what is the purpose of their...
-
You have been operating and growing your golf club for the last six (6) years. You are happy with the fact that all revenue streams (and as a result your share value) have continued to increase as...
-
Given the following HTML, write a simple bit of JavaScript code that will DELETE ALL OF THE TAGS ON THE PAGE. Quiz I'm a Heading I'm a paragraph I'm special I'm also a paragraph Footer! HINT: You'll...
-
Your company has been quite successful in sending employees on international assignments. As the HR Manager responsible for selecting such employees, present a report to the management of your...
-
You will be looking at a particular market in the economy. I will assign the market to you arbitrarily. Please look for at the end of this document to identify which market you will be responsible...
-
Explain how risk affects corporate financial strategy. Include the following in your answer: Business risk Credit risk Interest rate risk Political risk Reinvestment risk
-
Which provision could best be justified as encouraging small business? a. Ordinary loss allowed on $ 1244 stuck. b. Percentage depletion. c. Domestic production activates deductions. d. Interest...
-
Superacids are well established but superbases also exist and are usually based on hydrides of Group 1 and Group 2 elements. Write an account of the chemistry of superbases.
-
Calculate the equilibrium concentration of H 3 O + in a 0.10 M solution of butanoic acid (K a = 1.86 10 5 ). What is the pH of this solution?
-
In their paper The strengths of the hydrohalic acids (J. Chem. Educ., 2001, 78, 116), R. Schmid and A. Miah discuss the validity of literature values of the pK a values for HF, HCl, HBr, and HI. (a)...
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


