(a) Determine r H for the reactions of SrO and BaO with water, given that values...
Question:
(a) Determine ΔrHº for the reactions of SrO and BaO with water, given that values of ΔfHº(298 K) for SrO(s), BaO(s), Sr(OH)2(s), Ba(OH)2(s) and H2O(l) are −592.0, −553.5, −959.0, −944.7 and −285.5 kJ mol−1 respectively.
(b) Compare the values of ΔrHº with that for the reaction of CaO with water (eq. 12.5), and comment on factors contributing to the trend in values.
Equation 12.5
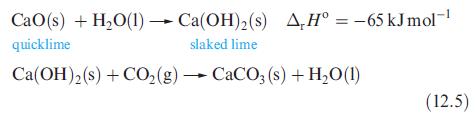
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





