(a) Draw resonance structures for CO, choosing only those that you think contribute significantly to the bonding....
Question:
(a) Draw resonance structures for CO, choosing only those that you think contribute significantly to the bonding.
(b) Figure 2.15a shows an MO diagram for CO. Two MOs are illustrated by schematic representations. Draw similar diagrams for the remaining six MOs.
Figure 2.15a
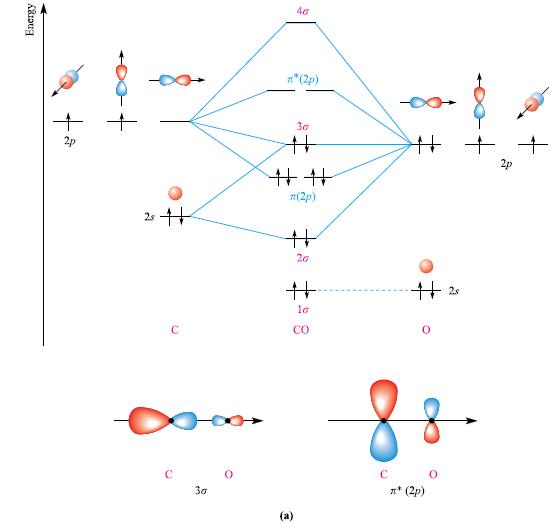
Transcribed Image Text:
Energy S 25+ с 30 40 n* (2p) 30 # ## z(2p) (a) 20 # la CO # ++ 25 8 0 CO n* (2p)
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw resonance structures for each of the following compounds: a. b. c. d. e. f. g. h. i. j. N.
-
Draw resonance structures for each of the following radicals: (a) (b) (c) (d)
-
Draw resonance structures for each of the following anions. a. b. c.
-
Which statement does NOT reflect a way that journals require authors to disclose potential or actual conflicts of interest? Group of answer choices Require researcher's Federal tax statements Require...
-
The following errors occurred in the accounting records of Coral Cove, Inc.: a. The company accountant recorded the receipt of cash for service revenue by debiting Cash for $470 instead of the...
-
In Exercises (a) Find the indefinite integral in two different ways. (b) Use a graphing utility to graph the antiderivative (without the constant of integration) obtained by each method to show that...
-
In companies in developing countries, imported management systems (like imported technology) can greatly boost efficiency and profits but not when they run counter to local customs and values. In...
-
Kopecky Industries Inc. is considering allocating a limited amount of capital investment funds among four proposals. The amount of proposed investment, estimated income from operations, and net cash...
-
El 1 de enero de 2015, South Carolina Development arrend un estacionamiento y tena la siguiente informacin: El monto del alquiler es de $210 000 por ao pagadero anualmente por adelantado. La vida...
-
Does VB theory indicate that the diatomic molecule He 2 is a viable species? Rationalize your answer.
-
Using VB theory and the Lewis structure model, determine the bond order in (a) H 2 , (b) Na 2 , (c) S 2 , (d) N 2 (e) Cl 2 . Is there any ambiguity with finding the bond orders by this method?
-
The total factory overhead for Lifestyle Furniture Company is budgeted at $600,000 for the year, divided between two departments: Fabrication, $420,000, and Assembly, $180,000. Lifestyle manufactures...
-
What is brand awareness for Jam & Daisies ? their leaning advantage, consideration advantage, choice advantages? 5. what is the recommendation of brand awareness? 6. What is Brand recognition? 7....
-
On August 1st, Custom Car Co's work in process inventory was $24900; its raw materials inventory was $6000; manufacturing overhead had a $1800 debit balance. Work in Process Subsidiary Data 8/1:...
-
Case: Castoro & Partners, CPAs is auditing Cloud 9 for the FY2023. Cloud 9 is a small public company and has been an audit client of Castoro & Partners since 2018. Materiality Methodology: Overall...
-
1)Solve the following differential equations by Undetermined Coefficient Method. dy dx dy - 4- 4+ 4y = 16x2e2x dx
-
Every year Monty Industries manufactures 8,600 units of part 231 for use in its production cycle. The per unit costs of part 231 are as follows: Direct materials Direct labor Variable manufacturing...
-
A realtor wanted to find a model that describes the asking price of houses in Greenville, South Carolina. She obtains a random sample of homes from the area. (a) Find the least-squares regression...
-
In a system with light damping (c < cc), the period of vibration is commonly defined as the time interval d = 2/d corresponding to two successive points where the displacement-time curve touches one...
-
When cobalt(II) salts are oxidized by air in a solution containing ammonia and sodium nitrite, a yellow solid, [Co(NO 2 ) 3 (NH 3 ) 3 ], can be isolated. In solution it is nonconducting; treatment...
-
Sketch the two structures that describe most five-coordinate complexes. Label the two different sites in each structure.
-
BINAP is a chelating diphosphine ligand shown below. Discuss the reasons for the observed chirality of BINAP, and of its complexes. PPh PPh
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


