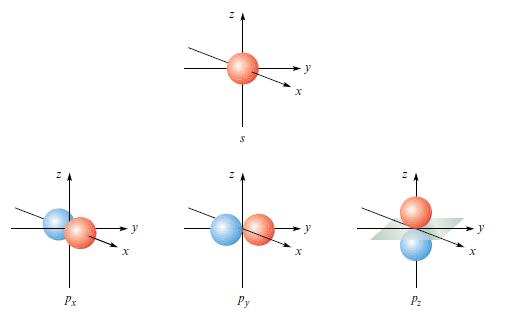
(a) How would Fig. 1.9 have to be modified to show boundary surfaces for the 2s and...
Question:
(a) How would Fig. 1.9 have to be modified to show boundary surfaces for the 2s and the 3p wavefunctions of a one-electron species?
(b) ‘The probability of finding the electron of a ground-state hydrogen atom at a distance r from the proton is at a maximum when r = 52.9 pm.’ Why is this statement compatible with the maximum in the value of R(r) at r = 0?
Figure 1.9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





