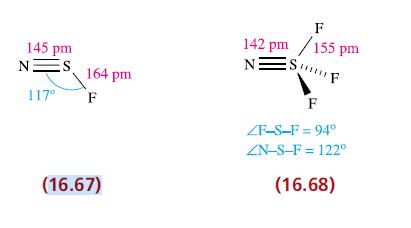
(a) Structures 16.67 and 16.68 show hypervalent sulfur in NSF and NSF 3 . Draw resonance structures...
Question:
(a) Structures 16.67 and 16.68 show hypervalent sulfur in NSF and NSF3. Draw resonance structures for each molecule that retains an octet of electrons around the S atoms, and account for the three equivalent S—F bonds in NSF3.
(b) The enthalpies of vaporization (at the boiling point) of H2O, H2S, H2Se and H2Te are 40.6, 18.7, 19.7 and 19.2 kJ mol−1. Give an explanation for the trend in these values.
(c) Which of the following compounds undergoes significant reaction when they dissolve in water under ambient conditions: Al2Se3, HgS, SF6, SF4, SeO2, FeS2 and As2S3? Give equations to show the reactions that occur. Which of these compounds is kinetically, but not thermodynamically, stable with respect to hydrolysis?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





