The electronic spectra of mixtures of CH 2 Cl 2 solutions (each 0.993 mmol dm 3 )
Question:
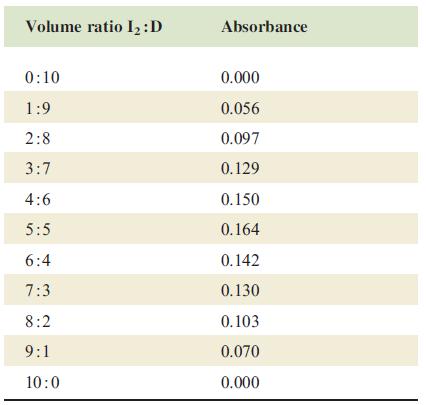
The electronic spectra of mixtures of CH2Cl2 solutions (each 0.993 mmol dm−3) of I2 and the donor D shown in the diagram after the table were recorded for different volume ratios of the two solutions. Values of the absorbance for the absorption at λmax = 308 nm are as follows:
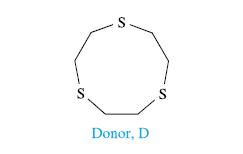
(a) Suggest how compound D might interact with I2.
(b) Use the data in the table to establish the stoichiometry of the complex formed between D and I2. Why can the absorbance data be used for this purpose?
(c) In the Raman spectrum of the complex, a band at 162 cm−1 is assigned to the I2 stretching mode. Explain why this value is shifted from that of 215 cm−1 for I2 itself.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





